Karthiksrao
- 66
- 0
Dear all,
In the wiki article about Debye solid :
https://en.wikipedia.org/wiki/Debye_model , in the section 'Another derivation', below Eq. 6, the following statement is provided:
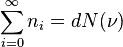
Here, I understand the right hand side, which is nothing but the density of states/modes at the frequency \nu.
I fail to understand how this can be equated to the total number of photons (particles) Sigma n_i
Is each mode/state equivalent to a particle ?
Thanks!
In the wiki article about Debye solid :
https://en.wikipedia.org/wiki/Debye_model , in the section 'Another derivation', below Eq. 6, the following statement is provided:
Here, I understand the right hand side, which is nothing but the density of states/modes at the frequency \nu.
I fail to understand how this can be equated to the total number of photons (particles) Sigma n_i
Is each mode/state equivalent to a particle ?
Thanks!