Ygggdrasil said:
The study has been criticized for some methodological flaws, however:
I agree, this study has several severe limitations.
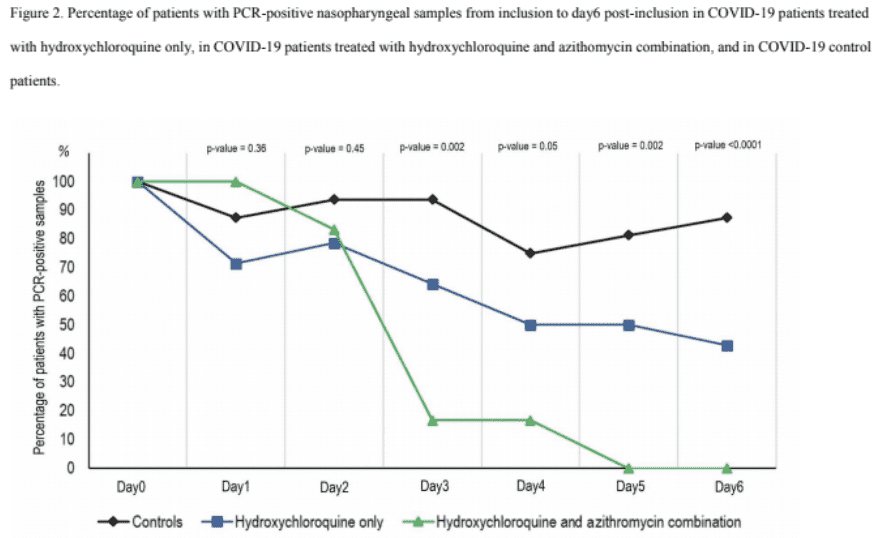
First and most importantly is the small sample size, only 6 patients were treated with the combination (Hydroxychloroquine and Azithromycin), and only 14 with the single drug (Hydroxychloroquine only). In drug studies this is a very, very, small sample. Unfortunately, small samples are plagued with a statistical effect that basically means that any effects that you do detect are inflated substantially by simple random statistical variation, simply by the fact that you did detect them. This is indicated by the 100% response rate, such a response rate is statistics, not reality.
Second is the “look elsewhere effect” which is another statistical phenomenon that inflates the significance of small samples when you are running multiple trials. Currently globally many doctors are trying many different treatments. With all of those doctors running small trials someone somewhere is essentially guaranteed to randomly get 6 responders in their treatment group. This one cannot be avoided in the context of coronavirus. But it means that the first paper on a successful treatment is not the important one. Look for the second paper from a different institution or multiple institutions with a much larger number of patients. That will be the informative paper.
Third, this was a non-randomized study design. That can cause problems, and importantly the patients who served as controls did not receive a placebo. That means that some of the effect will be the placebo effect.
Finally, six of the original Hydroxychloroquine group were excluded from the study. These are patients that began the treatment but whose data were not included in the study because they did not finish the course of treatment. Three did not finish because they were admitted to the ICU and one did not finish because they died. One did not finish because they left the hospital and another did not finish because of nausea. Of the four who died or went to the ICU, I think that it is fair to say that the treatment was ineffective, but their data was excluded.
Overall, I would say that this is a promising initial result and certainly warrants further investigation, but I would not consider it confirmed. If infected I would not make demands for these medicines, but I would definitely ask my doctor about it to see if there have been any follow-up studies demonstrating the effectiveness or in-effectiveness at another institution. Also, a discussion about the dosages and side-effects is important. If needed (God forbid), use it only after discussion with a trusted physician who knows your individual medical history. It may very well turn out to be effective, although I would expect true response rates closer to 50% than to 100%.
The CDC is likely to have the most up to date information, and does reference the above study. Hopefully all healthcare providers are reading the CDC guidance regularly. They seem to have the same attitude that I do. This is definitely worth further investigation, but it is not a recognized treatment yet:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html