Sometimes if the student said why he is looking for a answer to the particular question we could answer more to the point.
I think some important things to realize are the following:
The stoichiometric calculation is
quite close to right. What you have from half a mole of CO
2 per mole of NaOH is Na
2CO
3. For reference with a pK
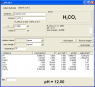
a2 I found of 10.33, for reference the pH of 1M Na
2CO
3 works out as 12.06! 2.6 M I get 12.37. But if you are working with other pK’s (not clear to me what ionic strength the above are at) maybe it is exactly 12?
How to get these results? Calculations with unbuffered solutions are just as tricky if not more so than with buffered ones so student should do some practice with them. (for another example see
https://www.physicsforums.com/showpost.php?p=3890835&postcount=7).
Write out conservation of mass equations and electroneutrality equations. Cross out all concentrations that are going to be negligible and hence show that in sodium carbonate solution [HCO
-3] = [OH
-]
Hence show that [OH
-] = √([CO
32-].K
w/K
a2)
And/or get an equation for [H
+] directly.
In conclusion I find that in 2.6 M Na
2CO
3 solution about 1 % of the carbonate is actually in the form bicarbonate HCO
3-. Now if we still want to get that down from pH 13.37 to 12.0 I find from Henderson-Hasselbalch (maybe there is a better way) that at pH12 about 2% is in form HCO
3 So I would have to remove about 1% of the CO2 to get from 12.37 to 12 I think. Or in the original terms of the problem the amount of CO2 I have to put in NaOH is about 99% of stoichiometric.
About the reservations made re ionic strength etc. OK. However calculations of amounts to be added or subtracted to change pH by a certain amount are reasonable, as much the same influences will be applying before and after such a change.
If still of interest it would be nice to see some actual calculations set out.
Of rather more useful interest than the pH of sodium carbonate is that of sodium bicarbonate. It is useful to realize that its pH is exactly half way between pK
a1 and pK
a2 of carbonic acid and so about 8.3, and a student should be sure he can show this.