sameeralord
- 659
- 3
Hello everyone,
This is a DIT molecule.
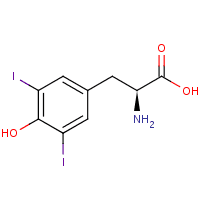
Now the book says there is a coupling reaction DIT + DIT ----> gives T4 molecule or tyroxine hormone
Now this is how T4 looks like
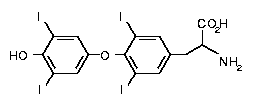
I don't understand how you can get this when there is a addition reaction between these two. Does coupling mean something else. Thank you
This is a DIT molecule.
Now the book says there is a coupling reaction DIT + DIT ----> gives T4 molecule or tyroxine hormone
Now this is how T4 looks like
I don't understand how you can get this when there is a addition reaction between these two. Does coupling mean something else. Thank you