Saladsamurai
- 3,009
- 7
Hello all!
I am reading through a combustion text and I am a little flustered by this notation:
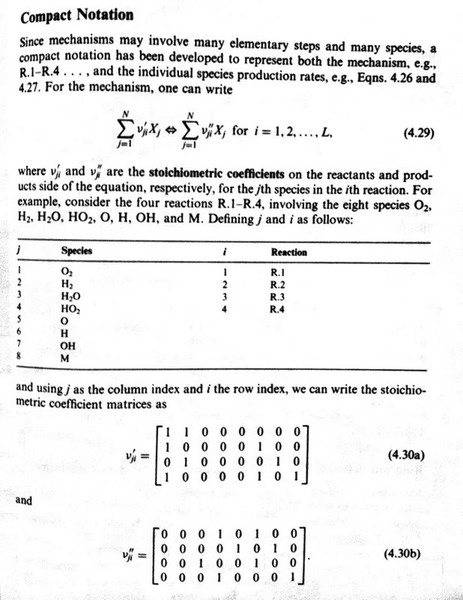
Am I correct in saying that the "coefficient matrices" {{\nu'_{ji}}} and {{\nu''_{ji}}} are not really "proper" matrices in the sense that there is not a vector that can multiply them that will result in the system of equations given by 4.29? Or is there? And I am just too tired to figure this out?
Also, the fact that the ij indices are reversed from normal convention (i.e., i = row index and j=column index) is really driving me batty!
I am reading through a combustion text and I am a little flustered by this notation:
Am I correct in saying that the "coefficient matrices" {{\nu'_{ji}}} and {{\nu''_{ji}}} are not really "proper" matrices in the sense that there is not a vector that can multiply them that will result in the system of equations given by 4.29? Or is there? And I am just too tired to figure this out?
Also, the fact that the ij indices are reversed from normal convention (i.e., i = row index and j=column index) is really driving me batty!