- #1
imconfused
- 6
- 0
(see image below) A solution made with 3mL of 0.1 M Fe(NO3)3, 3mL 0.1 M KSCN, and 35 mL of distilled water is poured into 4 test tubes. The color of the solution in each test tube is a blood red due to the formation of Fe(SCN)2+.
Test tube 1: a few drops of 0.1 M Fe(NO3)3 are added (the blood red color remains the same)
Test tube 2: a few drops of 0.1 M KSCN are added (the blood red color turns darker)
Test tube 3: 25 drops of 6 M NaOH are added (the blood red color turns to yellow)
Test tube 4: more distilled water is added (the blood red color becomes lighter in appearance)Okay, I'm confused when it comes to correlating my color observations of each test tube with Le Chatelier's principle. Test tube 1 and 2 throw me off because shouldn't they both turn darker because more Fe3+ and SCN ions are added which help produce more Fe(SCN)2+? Also, why does test tube 3 turn yellow, where does the yellow color come from? Test tube 4 is obvious because the concentration lessons. Please help, thanks you.
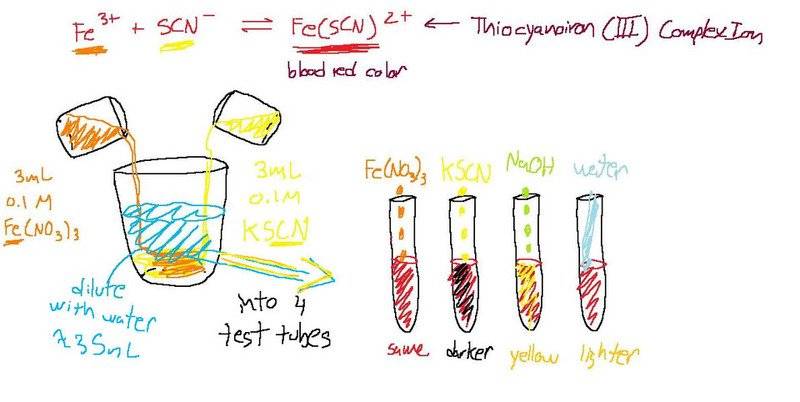
Test tube 1: a few drops of 0.1 M Fe(NO3)3 are added (the blood red color remains the same)
Test tube 2: a few drops of 0.1 M KSCN are added (the blood red color turns darker)
Test tube 3: 25 drops of 6 M NaOH are added (the blood red color turns to yellow)
Test tube 4: more distilled water is added (the blood red color becomes lighter in appearance)Okay, I'm confused when it comes to correlating my color observations of each test tube with Le Chatelier's principle. Test tube 1 and 2 throw me off because shouldn't they both turn darker because more Fe3+ and SCN ions are added which help produce more Fe(SCN)2+? Also, why does test tube 3 turn yellow, where does the yellow color come from? Test tube 4 is obvious because the concentration lessons. Please help, thanks you.
Last edited: