- #1
niranjan_learner
- 8
- 0
Homework Statement
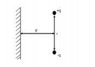
Two equal and opposite charges are placed at a distance r from each other. A large metallic sheet is placed at a distance d from them as shown in the figure. Due to the presence of the sheet, the attractive force between the charges along the direction joining them:
[Please Note: Figure attached]
1. decreases
2. increases
3. remains unchanged
4. decreases if r > d/2 and increases if r < d/2
Homework Equations
Coulombs equation for force between two charges.
The Attempt at a Solution
I attempted the problem by placing image charges -q and +q at a distance d to the left of the metallic plate. In that case the force between the real charges along the line joining them decreases. Is this solution acceptable?
Please review my answer. Thank you!