- #1
SpartanG345
- 70
- 1
There is such thing as a orthorhombic body centered crystal lattice. I am wondering why this is the case
see the image bellow, we can find a repeating pattern which has a smaller area.
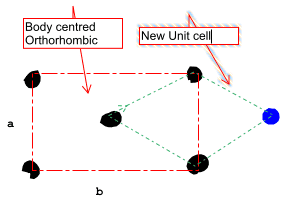
A unit cell
- must be selected such that it has the highest symmetry and the smallest area, however i do not see how the red unit cell has a higher symmetry. According to our lecture the red unit cell has a higher symmetry by 90 degrees, therefore a orthorhombic cubic has a body centered from
The green shape can be reflected vertically and horizontally, however the point of reflection where the axis of symmetry passes through is not the same is this why there is a higher symmetry for the red ( default) unit cell?
The blue atom is from a neighboring unit cell
see the image bellow, we can find a repeating pattern which has a smaller area.
A unit cell
- must be selected such that it has the highest symmetry and the smallest area, however i do not see how the red unit cell has a higher symmetry. According to our lecture the red unit cell has a higher symmetry by 90 degrees, therefore a orthorhombic cubic has a body centered from
The green shape can be reflected vertically and horizontally, however the point of reflection where the axis of symmetry passes through is not the same is this why there is a higher symmetry for the red ( default) unit cell?
The blue atom is from a neighboring unit cell
Last edited: