- #1
Corey Williams
- 5
- 0
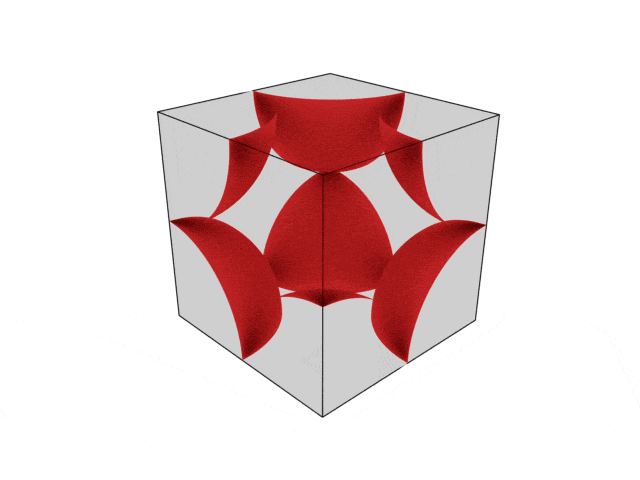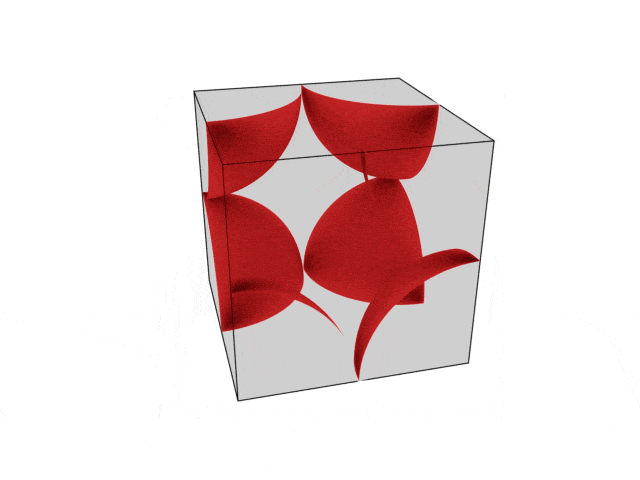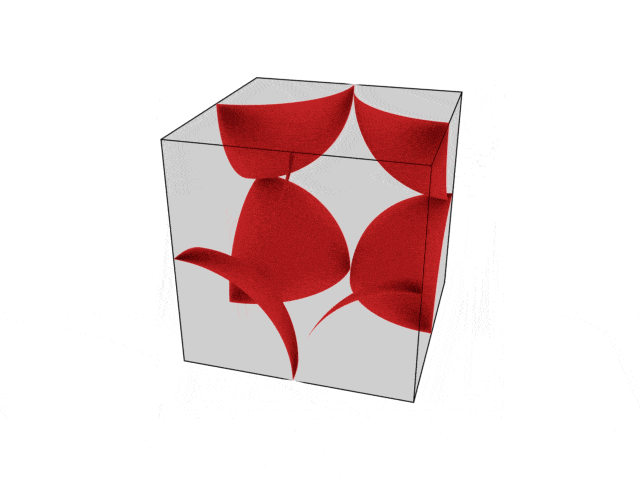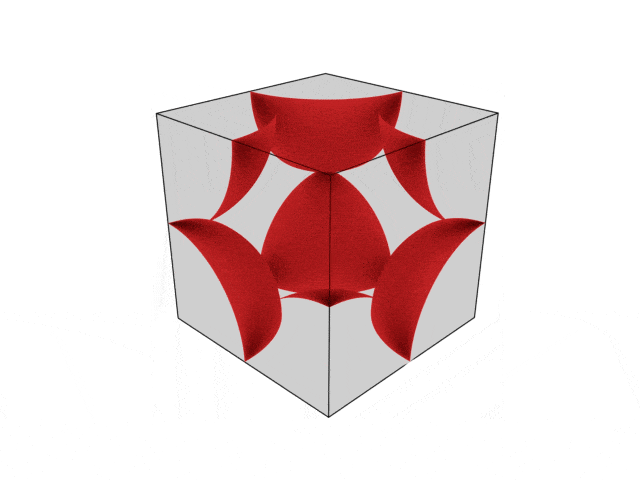
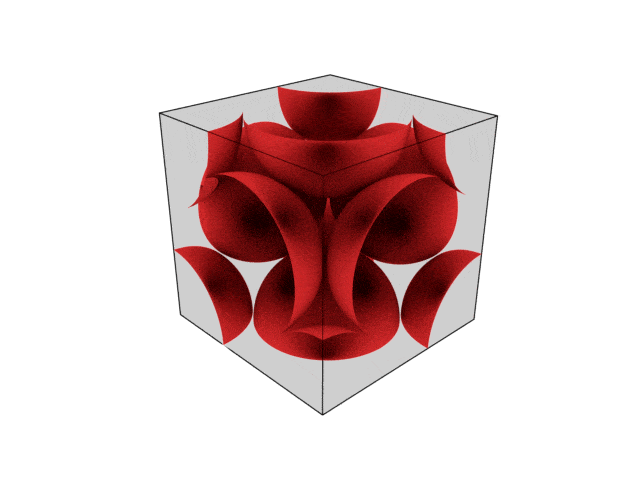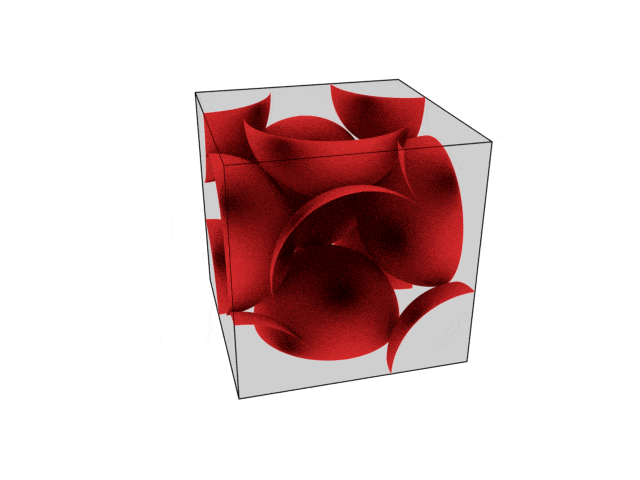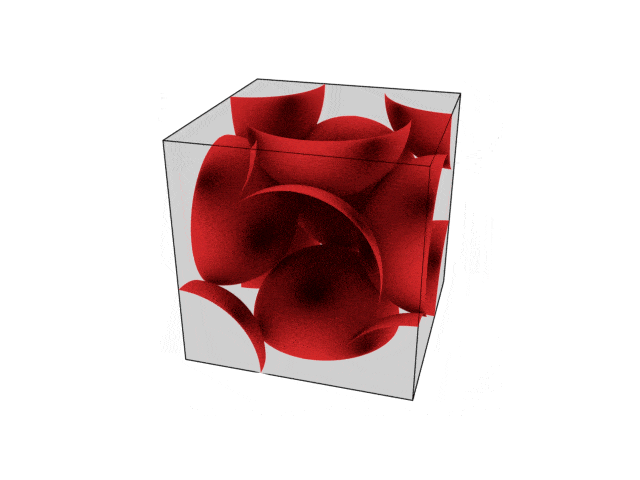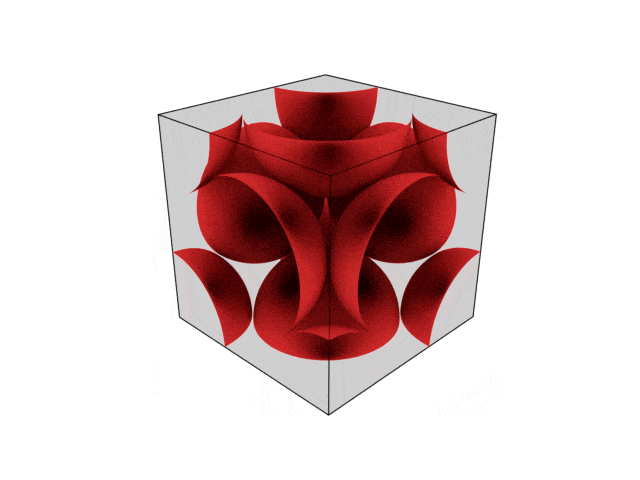
We were going over the structures of crystalline structures and the professor seemed to struggle to represent it nicely in 3D. I'm not sure if it is a common problem for students (I didn't think it was so hard to picture), but I decided to make a 3D representation of it that I thought would help.
Let me know what you think. Are they accurate enough? Are they helpful?
 https://www.physicsforums.com/attachments/112790
https://www.physicsforums.com/attachments/112790
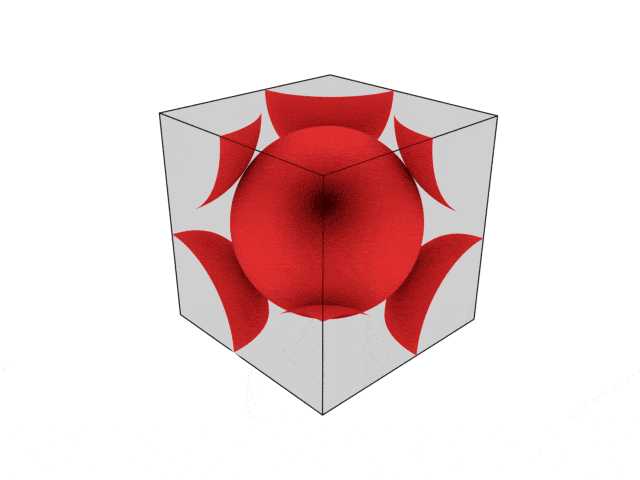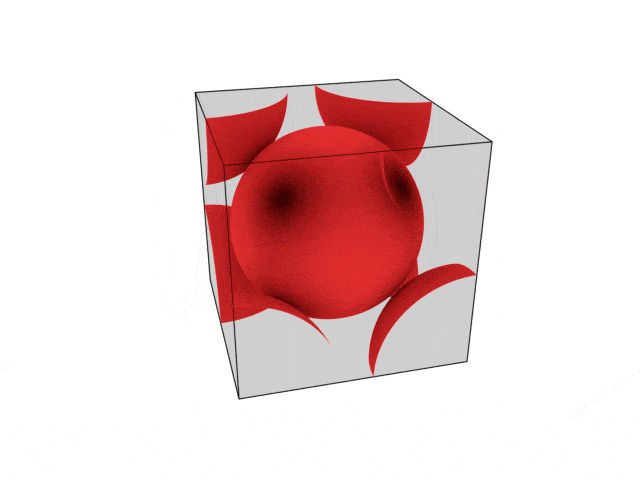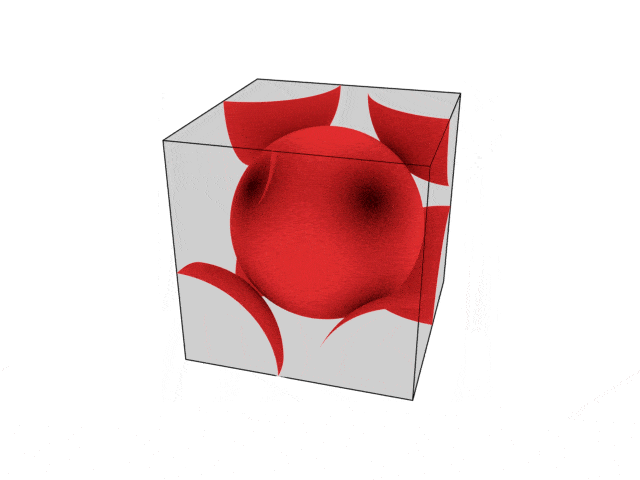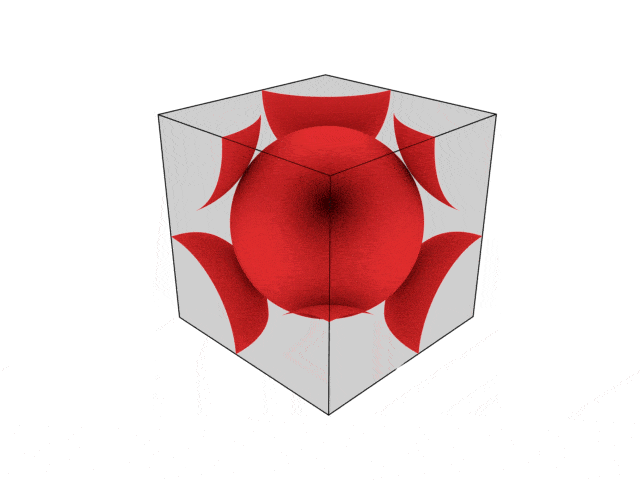
Let me know what you think. Are they accurate enough? Are they helpful?