Da Apprentice
- 57
- 0
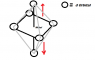
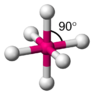
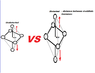
I've been told that the structure of Cu Nitrate is that of a octrahedral and appears similar to the picture attached. also that the distance at which the two verticle orbitals are away from each other varries the intensity of the blue colour, with the further the orbitals being away from each other the more intese the colour. I was just wondering why is this? and could someone please maybe further explain the images, i kind of know what they represnet but still don't fully understand them, for example are the orbitals pictured actually only the d orbitals and if so where are the others?
Thanks,
Thanks,