ACvub
- 9
- 0
Hello everybody,
I want to determine the extinction coefficients of Fe2+ and Fe3+ in glass.
There are literature data (e.g. Weyl's book "coloured glass"), so I know what kind of curves I should expect. As I am studying a slightly different soda-lime-silicate system, I want to recalculate the curves.
I have glasses with different Fe2+/Fe3+ ratio and total iron concentration [Fe].
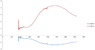
I have measured the absorbance of two glasses and I have solved a simple two equations system starting from Lambert-Beer law: A = Ʃ εCd
where A is absorption, ε is the extinction coefficient, C is the concentration and d is the thickness of my glass.
Unfortunately, I get negative values in one of the two extinction coefficient curves. Obviously, this doesn't make sense.
Anyone sees what is wrong in my reasoning? I can't figure it out.
Thank you very much in advance,
Mark
PS For a close look at the system I have made, see the attachment (.pdf)
I want to determine the extinction coefficients of Fe2+ and Fe3+ in glass.
There are literature data (e.g. Weyl's book "coloured glass"), so I know what kind of curves I should expect. As I am studying a slightly different soda-lime-silicate system, I want to recalculate the curves.
I have glasses with different Fe2+/Fe3+ ratio and total iron concentration [Fe].
I have measured the absorbance of two glasses and I have solved a simple two equations system starting from Lambert-Beer law: A = Ʃ εCd
where A is absorption, ε is the extinction coefficient, C is the concentration and d is the thickness of my glass.
Unfortunately, I get negative values in one of the two extinction coefficient curves. Obviously, this doesn't make sense.
Anyone sees what is wrong in my reasoning? I can't figure it out.
Thank you very much in advance,
Mark
PS For a close look at the system I have made, see the attachment (.pdf)