Discussion Overview
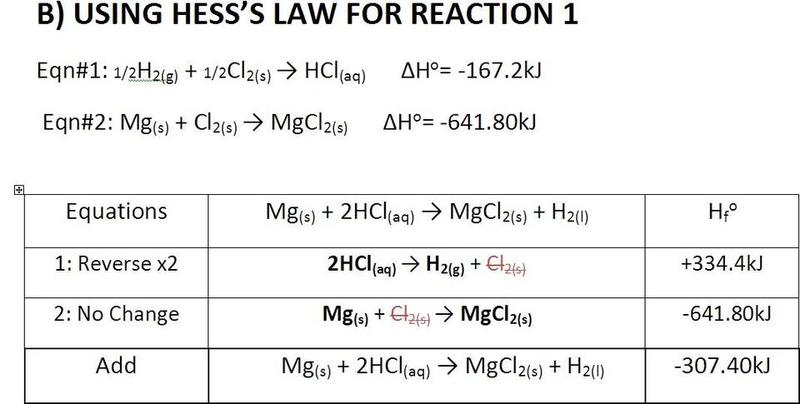
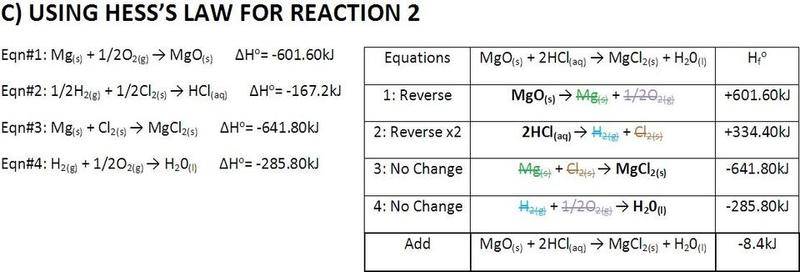
The discussion centers around the calculation of enthalpy for magnesium oxide (MgO) and hydrochloric acid (HCl) using Hess's Law. Participants are reviewing the calculations and seeking verification of the results, as well as sources for enthalpy values of molecules.
Discussion Character
- Technical explanation, Homework-related
Main Points Raised
- One participant requests verification of their enthalpy calculations, expressing uncertainty about the results.
- Another participant suggests that the calculations appear correct but notes that one answer should have an additional significant figure.
- A participant provides a link to a resource for finding standard enthalpy values, indicating that such tables may be available in textbooks as well.
Areas of Agreement / Disagreement
There is no explicit consensus on the correctness of the calculations, as one participant points out a potential issue with significant figures, while the original poster expresses uncertainty about their results.
Contextual Notes
The discussion does not clarify the specific enthalpy values used or the assumptions made in the calculations, leaving some aspects unresolved.