Cheman
- 235
- 1
Dissolving on a molecular level...
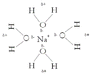
How do things dissolve eg in water? Thinking in terms of molecules, ions, etc, why does dissolving occur? Why do covalent molecules not dissolve in water but do in other covalents solvents, etc?
As much help as possible would be appreciated. :-)
Thanks.
How do things dissolve eg in water? Thinking in terms of molecules, ions, etc, why does dissolving occur? Why do covalent molecules not dissolve in water but do in other covalents solvents, etc?
As much help as possible would be appreciated. :-)
Thanks.