- #1
chingel
- 307
- 23
- TL;DR Summary
- Will a droplet forming at the end of the needle start climbing up the outer side of the needle to keep its contact angle? What shape will it form?
Let's say we are pushing water down a needle, the contact angle between the needle and the water is ##\mathrm{45°}##.
For simplicity let's assume there is no gravity.
As the water is initially moving down in the left image, the angle ##\theta=\mathrm{45°}## and the surface of the water is a part of a sphere with some fixed radius.
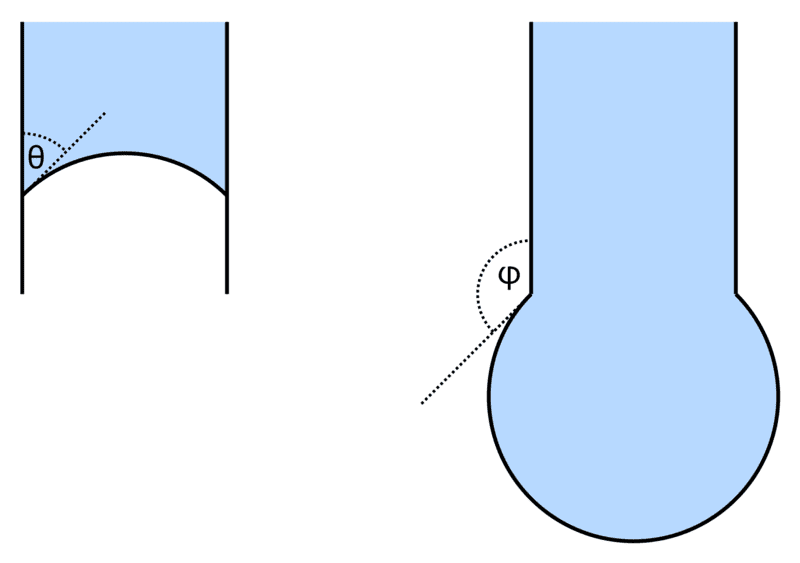
When the water moves to the end of the needle, we have ##\varphi=\mathrm{315°}##. As we push more water out, ##\varphi## keeps decreasing and the surface should always be a part of a sphere (or flat when ##\varphi=\mathrm{270°}##).
I would think that the droplet volume grows until it reaches the contact angle with the outer side of the needle (i.e. ##\varphi=\mathrm{135°}## in the figure on the right), and then I think the liquid should start moving up the outer side of the needle, since the contact line will be pulled up to keep the contact angle?
Since there is only one specific radius for a sphere with the given contact angle all around the needle, it seems the surface can't stay a sphere, contain more water and keep the ##\mathrm{45°}## contact angle at the same time, so what will it do if we push more water out?
For simplicity let's assume there is no gravity.
As the water is initially moving down in the left image, the angle ##\theta=\mathrm{45°}## and the surface of the water is a part of a sphere with some fixed radius.
When the water moves to the end of the needle, we have ##\varphi=\mathrm{315°}##. As we push more water out, ##\varphi## keeps decreasing and the surface should always be a part of a sphere (or flat when ##\varphi=\mathrm{270°}##).
I would think that the droplet volume grows until it reaches the contact angle with the outer side of the needle (i.e. ##\varphi=\mathrm{135°}## in the figure on the right), and then I think the liquid should start moving up the outer side of the needle, since the contact line will be pulled up to keep the contact angle?
Since there is only one specific radius for a sphere with the given contact angle all around the needle, it seems the surface can't stay a sphere, contain more water and keep the ##\mathrm{45°}## contact angle at the same time, so what will it do if we push more water out?
Last edited: