- #1
TheRedDevil18
- 408
- 1
Hi, I just want someone to explain this table to me please, I am totally confused especially with the Change(mol).
Question below:
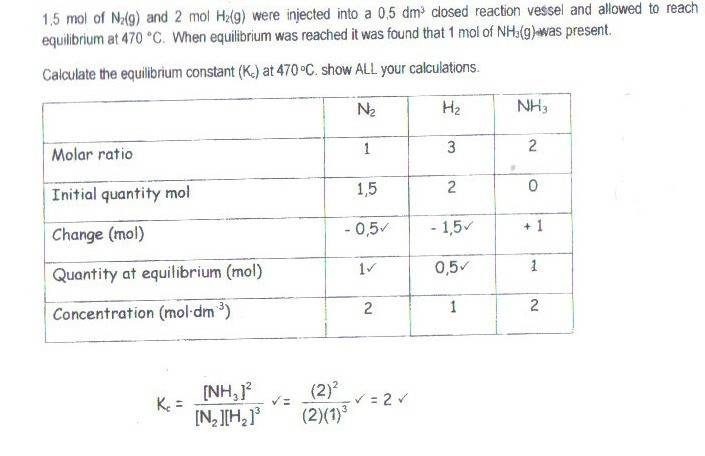
Question below:
The Equilibrium Constant Table Method is a mathematical technique used to determine the equilibrium constant for a chemical reaction. It involves setting up a table with the initial concentrations of reactants and products, as well as the change in concentration at equilibrium. The equilibrium constant can then be calculated using the concentrations at equilibrium.
The table is set up with the reactants and products listed on the left side, and the initial concentration, change in concentration, and equilibrium concentration for each species listed in separate columns. The top row of the table should have the labels "Reactant", "Initial Concentration", "Change in Concentration", and "Equilibrium Concentration".
The purpose of using this method is to determine the equilibrium constant for a chemical reaction. This constant is a measure of the extent to which a reaction will proceed and can be used to predict the concentrations of reactants and products at equilibrium.
The main assumptions made are that the reaction is taking place in a closed system, the reaction is at equilibrium, and the reaction is in a dilute aqueous solution. These assumptions allow for the use of the equilibrium constant expression and the ideal gas law to calculate the equilibrium constant.
Yes, this method is only applicable to reactions that are in equilibrium and that can be described by a simple equilibrium constant expression. It also assumes that the reaction is taking place in a dilute aqueous solution and at a constant temperature. Additionally, this method may not be accurate for reactions with large changes in concentration or when the initial concentrations are significantly different from each other.