- #1
rwooduk
- 762
- 59
Hi, are there any experimentalists here? I have gas in a cylinder. I have just been taking samples of gas from a tube connected to the outlet, but air is getting in the syringe.
So I went over to chemistry and got a round bottom flask with 3 inlets / outlets and a septum, so its like this:
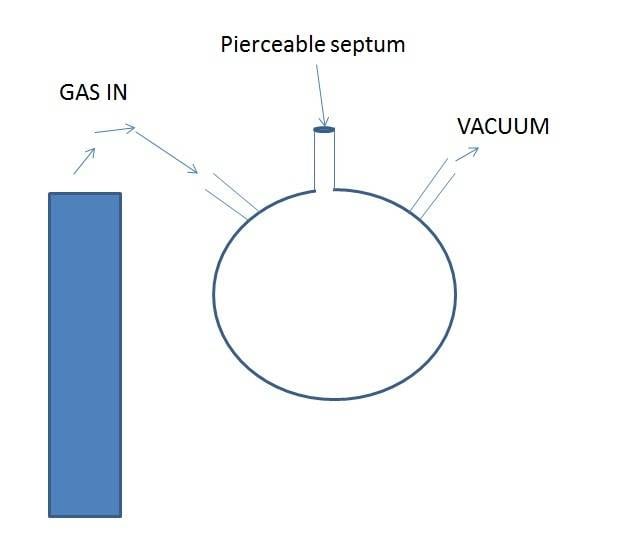
I evacuate the flask with the vacuum then flow the gas in, then after about a minute I pierce the septum with the GC syringe and take in some gas.
I am still getting air in the gas sample! Does anyone know a standard way of extracting gas from a cylinder (into a GC syringe) without air getting into it?
Any help would be really appreciated. I can order glassware etc etc but I don't know what specific equipment I need.
So I went over to chemistry and got a round bottom flask with 3 inlets / outlets and a septum, so its like this:
I evacuate the flask with the vacuum then flow the gas in, then after about a minute I pierce the septum with the GC syringe and take in some gas.
I am still getting air in the gas sample! Does anyone know a standard way of extracting gas from a cylinder (into a GC syringe) without air getting into it?
Any help would be really appreciated. I can order glassware etc etc but I don't know what specific equipment I need.
 Good luck! Sounds like your PhD is in secure hands.
Good luck! Sounds like your PhD is in secure hands.