somasimple
Gold Member
- 765
- 5
Hi,
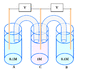
here is an example of a concentration cell
http://www.jce.divched.org/jcedlib/qbank/collection/conceptests/echem.html
1/ The final state is assumed when concentrations are equal in each compartment, right?
2/ Graphic evolution of the voltage is a decreasing curve, right?
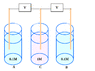
here is an example of a concentration cell
http://www.jce.divched.org/jcedlib/qbank/collection/conceptests/echem.html
1/ The final state is assumed when concentrations are equal in each compartment, right?
2/ Graphic evolution of the voltage is a decreasing curve, right?
Last edited by a moderator: