mathlearn
- 331
- 0
View attachment 6146
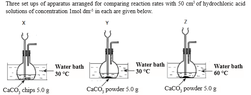
i.If all the calcium carbonate used in set up X was used up for the reaction, what is the amount of moles of carbon dioxide produced during the reaction ? (Ca = 40, C = 12, O = 16)
My progress:
Molar mass of CaCO3=(40+12+(16*3))gmol-1=100 gmol-1
Molar mass of CO2=12 + 16* 2 gmol-1=44 gmol-1
After that what must be done ?
Many Thanks :)
i.If all the calcium carbonate used in set up X was used up for the reaction, what is the amount of moles of carbon dioxide produced during the reaction ? (Ca = 40, C = 12, O = 16)
My progress:
Molar mass of CaCO3=(40+12+(16*3))gmol-1=100 gmol-1
Molar mass of CO2=12 + 16* 2 gmol-1=44 gmol-1
After that what must be done ?
Many Thanks :)