- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Fluid Flow Questions: Gas Flow Direction
- Thread starter jyakulis
- Start date
-
- Tags
- Flow Fluid Fluid flow
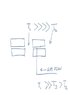
In summary, the conversation includes a discussion about a drawing that shows a system of pipes with gas flow and three temperatures, T1, T2, and T3. The purpose of the gas flow is to control the flow of molten metal through a gate system. The experts discuss the flow of gases and their movement between different temperatures. They also mention the addition of a hopper to the system and how it may affect the gas flow. The conversation ends with the expert offering to help the asker understand and solve their problem.
Physics news on Phys.org
- #2
Simon Bridge
Science Advisor
Homework Helper
- 17,874
- 1,661
Can't tell what that is a picture of.
There seem to be three temperatures - T1 seems to be between some rectangles, T2 seems to be to one side (presumably there is a temperature gradient of some kind in there? There is an intermediate T3 which does not seem to appear anywhere.
There is a label "gas flow" apparently along a line.
You need a clearer diagram.
There seem to be three temperatures - T1 seems to be between some rectangles, T2 seems to be to one side (presumably there is a temperature gradient of some kind in there? There is an intermediate T3 which does not seem to appear anywhere.
There is a label "gas flow" apparently along a line.
You need a clearer diagram.
- #3
jyakulis
- 9
- 0
Simon Bridge said:Can't tell what that is a picture of.
There seem to be three temperatures - T1 seems to be between some rectangles, T2 seems to be to one side (presumably there is a temperature gradient of some kind in there? There is an intermediate T3 which does not seem to appear anywhere.
There is a label "gas flow" apparently along a line.
You need a clearer diagram.
Let me clarify. Gas enters through an orifice labled gas flow. The gas entering through this temperature is somewhere between T1 and T2. T2 is at ambient atmosphere at close to room temperature. T1 is a chamber of fluid flow also at atmospheric pressure at very high temperatures. If the gas enters through the gas flow direction through the orifice at T3 which is between T1 and T2 where will it travel.
Thanks you.
- #4
Simon Bridge
Science Advisor
Homework Helper
- 17,874
- 1,661
What are the four blocks - cutaway of a system of pipes?
Does the interior also have gas in it or is it evacuated?
Is the system in equilibrium before the new gas is injected?
Gas already in the system will want to move away from the center (T1), but there will be some flow inwards. New gas injected at the place specified would tend to move with the ambient flow.
It "up" on the diagram is actually upwards, then the gas at T1 will prefer to exit through the top. Cool air comes in the bottom and the sides.
Does the interior also have gas in it or is it evacuated?
Is the system in equilibrium before the new gas is injected?
Gas already in the system will want to move away from the center (T1), but there will be some flow inwards. New gas injected at the place specified would tend to move with the ambient flow.
It "up" on the diagram is actually upwards, then the gas at T1 will prefer to exit through the top. Cool air comes in the bottom and the sides.
- #5
jyakulis
- 9
- 0
Simon Bridge said:What are the four blocks - cutaway of a system of pipes?
Does the interior also have gas in it or is it evacuated?
Is the system in equilibrium before the new gas is injected?
Gas already in the system will want to move away from the center (T1), but there will be some flow inwards. New gas injected at the place specified would tend to move with the ambient flow.
It "up" on the diagram is actually upwards, then the gas at T1 will prefer to exit through the top. Cool air comes in the bottom and the sides.
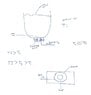
Don't want to give away too much but the blocks are two plates with holes in them acting as a valve. The valve is controlling the flow of molten metal. T3 is close to ambient temperature, but will be slightly hotter than T2 because of some heating while traveling to the injection point. Picture a groove around the center hole of the two blocks that distributes gas at T3 evenly around the hole. Interior of the hole has molten metal poring through it but it is open pore so, I would think it is at atmospheric pressure.
Not sure what you mean by equilibrium. I would say no it's more complex than that.
Evacuation of the interior is not possible. Is it creating a possible vacuum effect because due to poring the metal? I'm not sure.
- #6
Simon Bridge
Science Advisor
Homework Helper
- 17,874
- 1,661
Well then I cannot be precise.
If I imagine two plates with a hole in the middle, molten metal flows through the hole so no air can get in through the top or bottom, and the edges are open to the air - then some parts of the edge will have inflowing air and others outflowing with the exact places constantly changing unless the geometry is small enough for micro-cells to form.
What do you need to know for?
If I imagine two plates with a hole in the middle, molten metal flows through the hole so no air can get in through the top or bottom, and the edges are open to the air - then some parts of the edge will have inflowing air and others outflowing with the exact places constantly changing unless the geometry is small enough for micro-cells to form.
What do you need to know for?
- #7
- #8
jyakulis
- 9
- 0
I'm wondering what would happen if I play with the temperature of T3.
- #9
jyakulis
- 9
- 0
nevermind
by that i meant nevermind the comment not the question.
by that i meant nevermind the comment not the question.
Last edited:
- #10
jyakulis
- 9
- 0
But it gets complicated. At first pore maybe the plates are perfectly aligned. As life increases the holes become larger so they may be only partially open between subsequent pores creating air pockets as they are partially opened.
- #11
Simon Bridge
Science Advisor
Homework Helper
- 17,874
- 1,661
Nope - not at all clear.
You've just added a hopper - in the overhead view it looks not like there is a circular chamber around the hole the metal pours through - or maybe it's just a groove between two closely fitting plates. The angle that the gas is being injected suggests a vortex is added to whatever other motion their may be.
I have no idea. You'll just have to work it out from first principles... the hotter gas will be moving faster so it will tend to move away from the hot area ... cooler gas will try to move into replace it.
What are you hoping will happen? What is injecting the gas close to a column of molten metal supposed to achieve?
Bottom line though is that I am only here to help you understand (and phrase) your problems better so that you will know how to solve them. I am prepared to do that for free you understand... up to a point. If you are unwilling to tell me about your problem then I cannot help you.
You've just added a hopper - in the overhead view it looks not like there is a circular chamber around the hole the metal pours through - or maybe it's just a groove between two closely fitting plates. The angle that the gas is being injected suggests a vortex is added to whatever other motion their may be.
I have no idea. You'll just have to work it out from first principles... the hotter gas will be moving faster so it will tend to move away from the hot area ... cooler gas will try to move into replace it.
What are you hoping will happen? What is injecting the gas close to a column of molten metal supposed to achieve?
Bottom line though is that I am only here to help you understand (and phrase) your problems better so that you will know how to solve them. I am prepared to do that for free you understand... up to a point. If you are unwilling to tell me about your problem then I cannot help you.
- #12
jyakulis
- 9
- 0
Could you just explain how the flow of gases work first? Cool moves to hot? Or is it not that simple.
T3 is only very slightly hotter than T1 or very very close to the temperature of T1.
The boxes are what we call a slide gate plate system in the industry.
The hopper could either be a ladle for molten steel or what we call a tundish used for casting.
T3 is only very slightly hotter than T1 or very very close to the temperature of T1.
The boxes are what we call a slide gate plate system in the industry.
The hopper could either be a ladle for molten steel or what we call a tundish used for casting.
- #13
jyakulis
- 9
- 0
I'm guessing it further complicates matters that the gas coming in at T3 is argon and the gas at T2 is the atmosphere.
- #14
Simon Bridge
Science Advisor
Homework Helper
- 17,874
- 1,661
Not that simple.Could you just explain how the flow of gases work first? Cool moves to hot? Or is it not that simple.
In general - hot gas molecules move faster than cold ones so they tend to move away from the hot place. But that would reduce the pressure, sucking more gas in - which tends to be the cold gas. This van be chaotic (in the technical sense) but there are situations where you get circulation happening - which is called a cell.
Hot gas is less dense (because faster) so it tends to rise.
Think about how thunderheads form, or thermals, or how high explosive has a characteristic mushroom cloud.
All this is for some kind of open space - you can get gas to move in a narrow pipe fairly regularly - it does so by expanding from the hot end of the pipe though you'd usually pump it.
If the hot air escapes through a hole at the top of a chamber and you let cold air in through the bottom (it gets sucked in) you have a chimney effect.
A bunsen burner sucks air in through holes in the bottom of a pipe which has gas flowing through it under pressure. This is why I guessed that the injected gas will go with the ambient flow.
It is not easy.
lets see ... argon + molten metal going through holes: casting?
- #15
Integral
Staff Emeritus
Science Advisor
Gold Member
- 7,255
- 66
In investment casting or a sand mold I would think that air could flow thorough the mold. Like Simon I am having trouble interpreting your drawings.
I have done a fair amount of Aluminium investment casting.
I have done a fair amount of Aluminium investment casting.
FAQ: Fluid Flow Questions: Gas Flow Direction
1. What is the difference between laminar and turbulent flow?
Laminar flow is characterized by smooth, orderly movement of a fluid in a consistent direction. Turbulent flow, on the other hand, is characterized by chaotic movement of a fluid in different directions and velocities. Laminar flow is more common at lower velocities and in less viscous fluids, while turbulent flow is more common at higher velocities and in more viscous fluids.
2. How does gas flow direction affect fluid dynamics?
Gas flow direction can greatly impact the behavior of a fluid. For example, in a closed system, gas flowing in one direction can create a pressure gradient that causes the fluid to flow in the opposite direction. Additionally, changes in gas flow direction can result in changes in the speed and turbulence of the fluid, which can impact its overall dynamics and efficiency.
3. What factors influence the direction of gas flow?
The direction of gas flow is influenced by a variety of factors, including pressure differentials, temperature differentials, gravity, and external forces such as fans or pumps. Other factors, such as the shape and size of the fluid container, can also impact the direction of gas flow.
4. What are some common applications of studying gas flow direction?
Understanding gas flow direction is important in a variety of industries and research fields. For example, in the automotive industry, studying gas flow direction is crucial for designing more efficient engines. In the medical field, it is important for understanding the flow of gases in the respiratory system. It is also relevant in fields such as meteorology, aerodynamics, and chemical engineering.
5. How can gas flow direction be controlled or manipulated?
Gas flow direction can be controlled or manipulated through various methods, such as using valves or fans to change the pressure or direction of the gas. Changing the temperature or adding obstructions in the path of the gas can also alter its direction. In some cases, fluid dynamics simulations can also be used to predict and control gas flow direction in complex systems.
Similar threads
- Replies
- 7
- Views
- 1K
- Replies
- 28
- Views
- 1K
- Replies
- 9
- Views
- 1K
- Replies
- 35
- Views
- 3K
- Replies
- 18
- Views
- 1K
- Replies
- 48
- Views
- 3K
- Replies
- 160
- Views
- 7K
- Replies
- 20
- Views
- 2K
- Replies
- 9
- Views
- 1K
- Replies
- 6
- Views
- 783
Share: