- #1
kishtik
- 100
- 0
Last week, I've read about a plane (A300 I think) being lousy on a platform at a park, turning left and right, to where the wind comes. And I pondered about the energetic nature of the winds at that pause.
We could generate electric from wind, from air molecules' kinetic energy. They had kinetic energy because they moved from higher pressure to the lower. There were pressure differences because different areas were heated differently by the Sun. So I thought the Sun gave the air some potential energy by creating pressure differences.
Consider a syringe (without the string). Close its mouth with your finger and push its piston. Really you are giving it a PE. I think this system behaves like a spring.
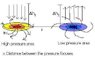
I pondered about how to calculate the potential energy in open air pressure differences (look at the attachment).
The difference between the final KE of a molecule and the initial KE of it had to give the KE gained from the pressure difference.
[tex]
KE_f - KE_i=KE_p
[/tex]
To calculate the final velocity, initial velocity must be known. No problem, we just need a thermometer.
[tex]
KE= \frac{1}{2} mv^2= \frac{3}{2} kT
[/tex]
Then we can return to our second formula. T_dif is the temperature difference.
[tex]
\frac{1}{2} mv_f^2-\frac{1}{2} mv_i^2= \frac{3}{2} k T_\textrm{dif}=KE_p
[/tex]
But when we say that two gases are at different pressures, it doesn't mean that their temperatures are different(PV=nRT).
I need a way to calculate KE_p without neccessity to the temperature difference.
After long minutes (err...) of thinking, I found that we could use the gravitational PE changes of the flows over the focuses (please look at the attachment again). I think Dh1 and Dh2 could be useful.
As you've seen these 5-min.-pause thoughts couldn't take me somewhere.
Next pause, I used a work approach.
If I could know how much work the wind does on a single molecule, I would feel like I was in heaven, but it didn't took more than a nanosecond to realize that this was impossible. First, all the molecules were traveling on different directions when the "air" was at rest. But no matter it was the high pressure area or other, the air was moving (as you can see at my stupid attachment) and this increased the sophistication of the subject and the confusion of the thinker. Second, I was only a high school student and did not know any way to find the average force on a molecule; more important, I knew nothing more than Bernoulli's effect about the fluid dynamics (which was very sophisticated, I learned from the tap in my bathroom).
Now, I know the subject is not very easy, but thinking about the nature became my lifestyle (perhaps since I read Richard Feynman).
www.geocities.com/sukreth/pressure.jpg
We could generate electric from wind, from air molecules' kinetic energy. They had kinetic energy because they moved from higher pressure to the lower. There were pressure differences because different areas were heated differently by the Sun. So I thought the Sun gave the air some potential energy by creating pressure differences.
Consider a syringe (without the string). Close its mouth with your finger and push its piston. Really you are giving it a PE. I think this system behaves like a spring.
I pondered about how to calculate the potential energy in open air pressure differences (look at the attachment).
The difference between the final KE of a molecule and the initial KE of it had to give the KE gained from the pressure difference.
[tex]
KE_f - KE_i=KE_p
[/tex]
To calculate the final velocity, initial velocity must be known. No problem, we just need a thermometer.
[tex]
KE= \frac{1}{2} mv^2= \frac{3}{2} kT
[/tex]
Then we can return to our second formula. T_dif is the temperature difference.
[tex]
\frac{1}{2} mv_f^2-\frac{1}{2} mv_i^2= \frac{3}{2} k T_\textrm{dif}=KE_p
[/tex]
But when we say that two gases are at different pressures, it doesn't mean that their temperatures are different(PV=nRT).
I need a way to calculate KE_p without neccessity to the temperature difference.
After long minutes (err...) of thinking, I found that we could use the gravitational PE changes of the flows over the focuses (please look at the attachment again). I think Dh1 and Dh2 could be useful.
As you've seen these 5-min.-pause thoughts couldn't take me somewhere.
Next pause, I used a work approach.
If I could know how much work the wind does on a single molecule, I would feel like I was in heaven, but it didn't took more than a nanosecond to realize that this was impossible. First, all the molecules were traveling on different directions when the "air" was at rest. But no matter it was the high pressure area or other, the air was moving (as you can see at my stupid attachment) and this increased the sophistication of the subject and the confusion of the thinker. Second, I was only a high school student and did not know any way to find the average force on a molecule; more important, I knew nothing more than Bernoulli's effect about the fluid dynamics (which was very sophisticated, I learned from the tap in my bathroom).
Now, I know the subject is not very easy, but thinking about the nature became my lifestyle (perhaps since I read Richard Feynman).
www.geocities.com/sukreth/pressure.jpg
Attachments
Last edited: