wildonion
- 5
- 2
- TL;DR Summary
- density, specific gravity, concentrations of salt water
I recently measured the specific gravity of salt water (NaCl) with a hand refractometer of solution to be 1.028 d 20/20 and 36-37 0/00 at 20 deg C. the d 20/20 and 0/00 are the units on the refractometer view screen. I am not sure if the 0/00 means percentage or g/kg.
I am trying to convert this into density (kg/m^3), concentration (mg/L), and (ppm TDS). I am confused on exactly how to do this.
Since the specific gravity is 1.028 that of freshwater, which is 1.000, then density would be 1028 kg/m^3 using the formula below?
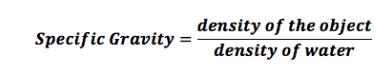
If the density of 1028 kg/m^3 and I convert units directly, I get something like:
1028 kg/m^3 * 1000000 mg/1kg * 1 m^3 / 1000 L = 1028000 mg/L or 1.028 kg/L
But I have been reading that sea water of approximate density of 1.025 kg/L has approximately 3.5% salt content, 35 g/kg, or 35 000 ppm AND that ppm and mg/L are the same with liquids. So I am really confused why 1028000 mg/L is so much larger. Can anyone point me in the right direction?
I know there is a temperature dependence here too, but I am trying to understand if I am in the ballpark for the concentration value.
I am trying to convert this into density (kg/m^3), concentration (mg/L), and (ppm TDS). I am confused on exactly how to do this.
Since the specific gravity is 1.028 that of freshwater, which is 1.000, then density would be 1028 kg/m^3 using the formula below?
If the density of 1028 kg/m^3 and I convert units directly, I get something like:
1028 kg/m^3 * 1000000 mg/1kg * 1 m^3 / 1000 L = 1028000 mg/L or 1.028 kg/L
But I have been reading that sea water of approximate density of 1.025 kg/L has approximately 3.5% salt content, 35 g/kg, or 35 000 ppm AND that ppm and mg/L are the same with liquids. So I am really confused why 1028000 mg/L is so much larger. Can anyone point me in the right direction?
I know there is a temperature dependence here too, but I am trying to understand if I am in the ballpark for the concentration value.