- #1
BWV
- 1,465
- 1,781
Been reading basic stuff about the astrophysics behind the prevalence of various elements, curious at what rate this changes over time. The Milky Way is something like 14 billion years old. At first it was all H and He then heavier elements were created and distributed through supernovae.
Questions
a) How long did it take before the prevalence of the elements looked close to its current state? there was about 10B years between the formation of the galaxy and our solar system. Was the galaxy significantly different, say, 8B years ago? Is this maybe a potential answer to the Fermi Paradox? How about 10B years from now?
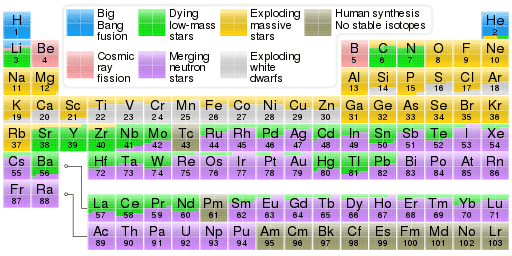
b) I had thought is was exclusively supernovae that created heavier elements, but the chart below on the wikipedia entry lists merging neutron stars as the most common source of heavier elements, how does that work? would have guessed that merging neutron stars, depending on the mass, either created a bigger neutron star or a black hole
Questions
a) How long did it take before the prevalence of the elements looked close to its current state? there was about 10B years between the formation of the galaxy and our solar system. Was the galaxy significantly different, say, 8B years ago? Is this maybe a potential answer to the Fermi Paradox? How about 10B years from now?
b) I had thought is was exclusively supernovae that created heavier elements, but the chart below on the wikipedia entry lists merging neutron stars as the most common source of heavier elements, how does that work? would have guessed that merging neutron stars, depending on the mass, either created a bigger neutron star or a black hole
