lioric
- 335
- 26
I know that if I multiply e^3/2 (e= 1.602x10^-19) to the unit below I can change it to eV
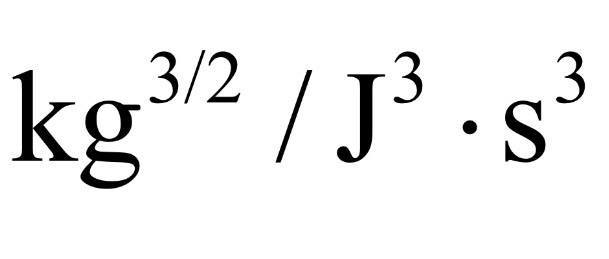
Can somebody help me I don't know why
Can somebody help me I don't know why