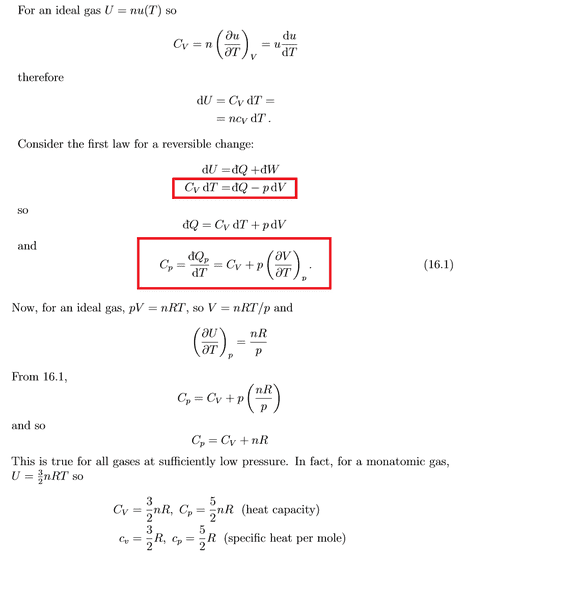
Clara Chung said:
I think that dU = Cv dT can only be used when the volume of the gas is constant.
Similarly, Cp = dQ/dT can only be used when the pressure is constant.
Such as
We have Cv=dQ/dT
and ΔU=Q+W
dU=Q+pdV
Therefore,
dU/dT = dQ/dT only when dV is zero
Why am I wrong? Thank you so much for your help. The original proof is below.
View attachment 219513
The reason you are struggling so much with this is that it was taught to you incorrectly in the first place. In freshman physics, they only talked about solid systems or liquid systems (which are basically of constant volume), and they taught us that the amount of heat transferred to the material could be expressed in terms of the heat capacity of the system by ##Q = mC\Delta T##. But in all these constant volume systems, no work was being done.
Now, we are in thermodynamics, and we learn that, when work is being done, the heat Q depends on the path of the process while the heat capacity is supposed to be a physical property of the material that depends only the state of the system, and not on the path for the process. Not only that, but, when work is done, the heat is no longer given by ##Q=mC\Delta T##,, unless we change the definition of C, depending on whether the process is of constant volume, constant pressure, or something else. So, what now. How can we correct all this, and still preserve some semblance of the heat capacity understanding that we learned in freshman physics?
What follows is how we do it. We have learned in thermodynamics about the thermodynamic functions internal energy U and enthalpy H, and how these are used in conjunction with the first law of thermodynamics. These functions are physical properties of the material that depend only on its thermodynamic state. This is exactly the characteristic that we would like to preserve for the heat capacity. So, we now have developed the following new definitions of heat capacity (In our subsequent development, we will be referring the to properties
per mole or
per unit mass):
$$C_v=\left(\frac{\partial U}{\partial T}\right)_V$$
$$C_p=\left(\frac{\partial H}{\partial T}\right)_P$$
For a constant volume change or a constant pressure change, these definitions will give results that are consistent with the previous freshman physics results. However, they are much more general than that. Not only this, but the heat capacities are now clearly physical properties of the material that depend only on the thermodynamic state.
Later, you will learn more generally that the differential changes in internal energy and enthalpy of materials depend not only on temperature, but also on pressure and volume according to the relationships:
$$dU=C_vdT-\left[P-T\left(\frac{\partial P}{\partial T}\right)_V\right]dV$$
$$dH=C_vdT+\left[V-T\left(\frac{\partial V}{\partial T}\right)_P\right]dP$$
These relationships apply to real gases. Note that, for an ideal gas, PV=RT, and the second terms in each of these expressions is identically equal to zero. This means that, both Cv and Cp are functions only of temperature for an ideal gas.
We also know that, for an ideal gas,
$$dH=dU+d(PV)$$
So, using our new definitions, we find for an ideal gas that $$C_pdT=C_vdT+dPV)=C_vdT+RdT$$So it follows immediately that $$C_p=C_v+R$$