Hyo X
- 101
- 11
I have electron microscope images of structures of varying periodicity, ranging from highly crystalline to highly amorphous. It is relatively straightforward to take FFTs of these images, but what i want to do is extract a quantitative value to characterize crystallinity and compare it between samples.
The easier question is, what kind of information can I extract from 2D FFTs about the structures?
The more difficult question is: how can I quantitatively compare FFTs between different images?
I have some ideas, but would appreciate suggestions. These are two example FFTs.
Image1

---
Image 2
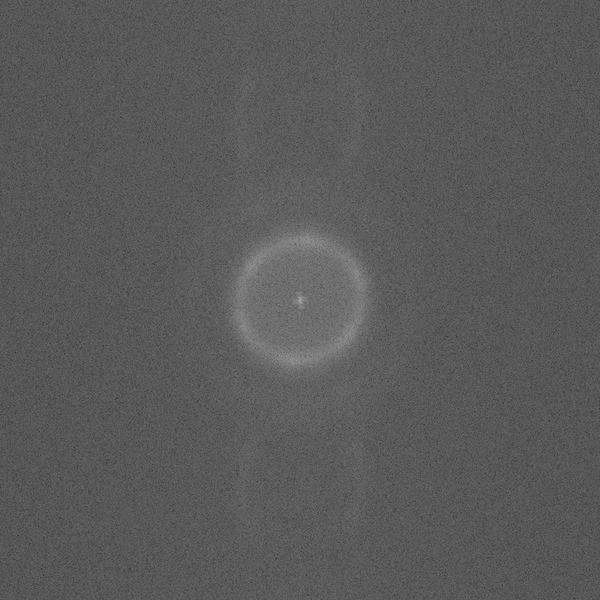
The easier question is, what kind of information can I extract from 2D FFTs about the structures?
The more difficult question is: how can I quantitatively compare FFTs between different images?
I have some ideas, but would appreciate suggestions. These are two example FFTs.
Image1
---
Image 2