You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
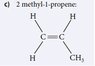
Is 2-Methyl-1-Propene the Correct Naming for this Molecule?
- Thread starter quicksilver123
- Start date
-
- Tags
- Molecule
AI Thread Summary
The discussion centers on the correct naming of a molecule with a three-carbon chain and a double bond. The consensus is that the correct name is propene, as the prefix "1-" is unnecessary. The term "methylethene" is clarified to be incorrect, as it does not accurately represent the structure. The conversation also touches on "2-methylpropene," which has four carbons and is also known as isobutylene. The participants emphasize the importance of proper nomenclature in organic chemistry and provide a reference link for further information.
Chemistry news on Phys.org
Traxfam
- 7
- 1
propene is the correct name the 1 is not necessary, more commonly it is known as propylene
quicksilver123
- 173
- 0
Thanks. So methylethene=propene?
Traxfam
- 7
- 1
Not quite, it is just propene 3 carbons one double bond. I am not sure where the methyl propene came from. 2-Methyl propene would have four carbons it is also known as isobutylene, here is a link
http://en.wikipedia.org/wiki/Isobutylene
http://en.wikipedia.org/wiki/Isobutylene
I want to test a humidity sensor with one or more saturated salt solutions. The table salt that I have on hand contains one of two anticaking agents, calcium silicate or sodium aluminosilicate. Will the presence of either of these additives (or iodine for that matter) significantly affect the equilibrium humidity?
I searched and all the how-to-do-it guides did not address this question. One research paper I found reported that at 1.5% w/w calcium silicate increased the deliquescent point by...
https://cen.acs.org/synthesis/c-h-activation/Chemists-finally-know-palladium-beats/103/web/2025/09
I'm trying to find a cheap DIY method to etch holes of various shapes through 0.3mm Aluminium sheet using 5-10% Sodium Hydroxide. The idea is to apply a resist to the Aluminium then selectively ablate it off using a diode laser cutter and then dissolve away the Aluminium using Sodium Hydroxide. By cheap I mean resists costing say £20 in small quantities.
The Internet has suggested various resists to try including...
Enamel paint (only survived seconds in the NaOH!)
Acrylic paint (only...
Similar threads
- Replies
- 3
- Views
- 1K
- Replies
- 1
- Views
- 3K
- Replies
- 1
- Views
- 2K
- Replies
- 3
- Views
- 5K
- Replies
- 3
- Views
- 7K
- Replies
- 1
- Views
- 2K
- Replies
- 2
- Views
- 2K
- Replies
- 1
- Views
- 4K
- Replies
- 8
- Views
- 8K
- Replies
- 6
- Views
- 3K
Hot Threads
Recent Insights
-
Insights Why Entangled Photon-Polarization Qubits Violate Bell’s Inequality
- Started by Greg Bernhardt
- Replies: 26
- Quantum Interpretations and Foundations
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 11
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 5
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 105
- General Math