Deathcrush
- 36
- 0
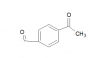
Does anyone know if an aldehyde, specifically, acetaldehyde behaves as a good nucleophile? I'm trying to insert an aldehyde in an aromatic compound through an aromatic nucleophilic substitution, any information or source to pKa data would also be appreciated, thanks!