Pushoam said:
View attachment 214876
The above equation comes from the 1st law of thermodynamics ( ##\Delta U = \Delta Q + \Delta W ##)which is valid for ( I think ) both reversible and irreversible processes.
For reversible process, U as a function of V and T and dW = - p dV are defined. So, the above equation is valid only for reversible process. Right ?
For an irreversible path, p and T are not uniform within the system, and viscous stresses contribute to the force per unit area that the gas exerts on the surroundings, so the equation of state can not be used to establish the force on the surroundings or the work. Also, for both an irreversible path or a reversible path, the "volumetric" work done by the system on the surroundings can be determined by integrating ##p_{ext}dV##, where ##p_{ext}## is the force per unit area exerted by the surroundings on the system (if this force is somehow known or specified). In the case of a reversible path, ##p_{ext}## also matches the pressure of the gas within the system p calculated from the equation of state.
Is this because the above eqn deals with the infinitesimal change and the difference between two closely neighboring eqbm states is infinitesimal?
Yes.
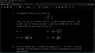
The book says,
View attachment 214876
Here, ## C_V ## is defined as ## C_V = \left ( \frac {\partial Q } {\partial T } \right) _{V} ## and then on calculation it is found that ## C_V = \left ( \frac {\partial U } {\partial T } \right) _{V} ##.
What I have understood from your statement as ## C_V ## is defined as ## C_V = \left ( \frac {\partial U } {\partial T } \right) _{V} ##.
Is this correct?
I completely disagree with how this is presented in your book, and, as justification for this disagreement, I cite your present state of confusion.
In freshman physics, they taught us that ##Q=C\Delta T##, where they called C the heat capacity of the material and Q the heat transferred to the material. All the problems to which this was applied were ones in which no work was done.
Now we arrive at thermodynamics, and we encounter situations where work can be done on the surroundings. And immediately we encounter problems with using this old definition because Q is supposed to be (exclusively) the heat transferred from the surroundings to the system, while C is supposed to be a physical property of the material. And, when work is being done, Q is not longer equal to ##C\Delta T##. So, in thermodynamics, we find it necessary to discard the old definition of C in terms of Q, and adopt a new definition in which we consistently get the right answer, whether or not work is being done, while, at the same time reducing to the old definition when work is not being done. This new definition is:
$$C_v=\left(\frac{\partial U}{\partial T}\right)_V$$and $$C_p=\left(\frac{\partial H}{\partial T}\right)_P$$These new definitions clear up all the difficulties with Q being energy in transit (specifically for a process) while C is a physical property of the material (independent of any process).
For any reversible process,
## \Delta U ## between any two states or dU between any two close states is well defined independently of path.
## \Delta Q ## between any two states or dQ between any two close states is well defined for any given path.
There are an infinite number of ##\Delta Q##s or dQs between any two close states for the infinite number of paths between the two states. But there is only one ##\Delta U## or dU. So, clearly, Q is not a unique function of state. It is a function only of path. And the path cannot be specified giving the
system temperature and pressure as a function of time along the path, because these are not uniform within the system for an irreversible path.
So, U is well defined as a function of state variables independently of the path.
Yes.
And Q is well defined as a function of the state variables for a given path. Isn’t it so?
No.
View attachment 214880
The book says that for reversible process both TdS and pdV are differrential of state fn. So, their definite integral i.e Q and W respectively should also be state fn. But, it is said that Q and W are not state fn. This is where I am stuck.
Q is not the definite integral of TdS and W is not the definite integral of PdV. This is only true for a reversible path. For an irreversible process, we can only know T at the boundary of the system (typically an ideal reservoir temperature, ##T_R##), and, for such a process, the most that we can say is that $$\int{\frac{dQ}{T_R}}\lt\Delta S$$where ##\Delta S## is the
change in entropy between the initial and final thermodynamic equilibrium states of the system (i.e., assuming that the final state is an equilibrium state). And, for both reversible and irreversible processes, $$W=\int{p_{ext}dV}$$.
Here is an article I wrote for Physics Forums Insights that I wrote a couple of years ago that might help:
https://www.physicsforums.com/insights/understanding-entropy-2nd-law-thermodynamics/