You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
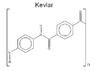
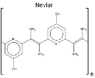
Is Nevlar, a New Polymer Design, Stronger than Kevlar?
- Thread starter bomba923
- Start date
AI Thread Summary
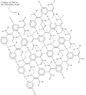
The discussion centers on the comparison between Nevlar, a polymer designed by the original poster, and Kevlar, a well-known high-strength material. The poster expresses skepticism about Nevlar's strength compared to Kevlar, citing concerns about the bond orientations and their effectiveness. They acknowledge that their bond representations may not be to scale, which could affect perceptions of strength. The conversation invites suggestions for molecular modeling software to create better visual representations of Nevlar's structure, aiming to clarify its potential strength relative to Kevlar.
Chemistry news on Phys.org
3trQN
- 337
- 1
I don't think it will be stronger, the orientations of the bonds (and what i would expect bond orbital overlaps to be like by mental picture) doesn't look nearly as optimum as in kevlar. But what do i know...
bomba923
- 759
- 0
Well, the bonds in my third image aren't exactly "drawn to scale" (so a mental picture might look different...). If you can suggest any molecular modeling freeware, I can post a much better image--
Why so?3trQN said:orientations of the bonds ... doesn't look nearly as optimum as in kevlar
Last edited:
It seems like a simple enough question: what is the solubility of epsom salt in water at 20°C? A graph or table showing how it varies with temperature would be a bonus. But upon searching the internet I have been unable to determine this with confidence. Wikipedia gives the value of 113g/100ml. But other sources disagree and I can't find a definitive source for the information. I even asked chatgpt but it couldn't be sure either. I thought, naively, that this would be easy to look up without...
Do the published values of Enthalpy include the work done against a constant pressure, e.g., the atmosphere? (I am not a chemist). I am reviewing enthalpy and entropy as part of the statistical mechanics applied to transistors. I assume, from my reading, that the work done would mostly apply to reactions involving gasses.
I was introduced to the Octet Rule recently and make me wonder, why does 8 valence electrons or a full p orbital always make an element inert?
What is so special with a full p orbital?
Like take Calcium for an example, its outer orbital is filled but its only the s orbital thats filled so its still reactive not so much as the Alkaline metals but still pretty reactive.
Can someone explain it to me?
Thanks!!
Similar threads
- Replies
- 1
- Views
- 5K
- Replies
- 7
- Views
- 12K
- Replies
- 8
- Views
- 2K
- Replies
- 1
- Views
- 2K
- Replies
- 2
- Views
- 5K
- Replies
- 2
- Views
- 627
- Replies
- 4
- Views
- 2K
- Replies
- 6
- Views
- 12K
- Replies
- 7
- Views
- 2K
- Replies
- 7
- Views
- 6K
Hot Threads
Recent Insights
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 7
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 0
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 93
- General Math
-
Insights Why Vector Spaces Explain The World: A Historical Perspective
- Started by fresh_42
- Replies: 0
- General Math