- #1
MexChemE
- 237
- 55
Hello, PF!
I'm currently brushing up my fluid mechanics and came across some questions while studying the compressible flow of an ideal gas using Bernoulli's equation. First, consider incompressible flow in the following system
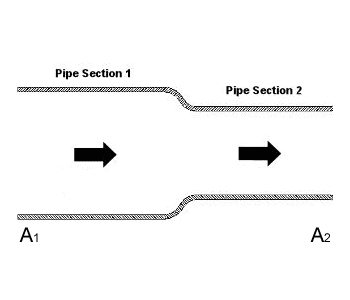
Neglecting any changes in elevation, the Bernoulli equation for this system is
[tex]P_1 - P_2 = \frac{1}{2} \rho \left( v_2^2 - v_1^2 \right)[/tex]
And the continuity equation states
[tex]A_1 v_1 = A_2 v_2[/tex]
Now, since [itex]A_1 > A_2[/itex], then [itex]v_2 > v_1[/itex], therefore [itex]P_1 > P_2[/itex]. An increase in velocity results in a decrease in pressure. This is Bernoulli's principle.
Now consider the isothermal flow of an ideal gas through the same system. Bernoulli's equation in this situation is
[tex]\frac{RT}{M} \ln{\frac{P_1}{P_2}} = \frac{1}{2} \left( v_2^2 - v_1^2 \right)[/tex]
I'm going to call this equation A. This equation has units of energy per unit mass. Now, the continuity equation for this case is
[tex]\rho_1 A_1 v_1 = \rho_2 A_2 v_2[/tex]
Or, using the ideal gas law
[tex]P_1 A_1 v_1 = P_2 A_2 v_2[/tex]
Now, according to equation A, if [itex]v_2 > v_1[/itex], then [itex]P_1 > P_2[/itex], so Bernoulli's principle holds. However, this is where I'm having a little bit of trouble, in the incompressible case, the decrease in flow area is what caused the increase in velocity (and will always do, according to the continuity equation). In the compressible case (and again, according to the corresponding continuity equation) a decrease in flow area may not always result in an increase in velocity, since we have to take into account the change in density/pressure of the gas. In other words, for this particular system, even if [itex]A_1 > A_2[/itex] will always be true, [itex]v_1[/itex] may be greater or smaller than [itex]v_2[/itex], depending on the nature of the gas and the temperature of the system. Is this right?
Are there any rules of thumb or other relationships between flow area and velocity for compressible flows? I know this might seem like a simple detail but it really caught me off guard.
Also, for the incompressible case, is it okay to use gauge pressures in calculations involving the Bernoulli equation or any other form of the mechanical energy balance?
Thanks in advance for any input!
I'm currently brushing up my fluid mechanics and came across some questions while studying the compressible flow of an ideal gas using Bernoulli's equation. First, consider incompressible flow in the following system
Neglecting any changes in elevation, the Bernoulli equation for this system is
[tex]P_1 - P_2 = \frac{1}{2} \rho \left( v_2^2 - v_1^2 \right)[/tex]
And the continuity equation states
[tex]A_1 v_1 = A_2 v_2[/tex]
Now, since [itex]A_1 > A_2[/itex], then [itex]v_2 > v_1[/itex], therefore [itex]P_1 > P_2[/itex]. An increase in velocity results in a decrease in pressure. This is Bernoulli's principle.
Now consider the isothermal flow of an ideal gas through the same system. Bernoulli's equation in this situation is
[tex]\frac{RT}{M} \ln{\frac{P_1}{P_2}} = \frac{1}{2} \left( v_2^2 - v_1^2 \right)[/tex]
I'm going to call this equation A. This equation has units of energy per unit mass. Now, the continuity equation for this case is
[tex]\rho_1 A_1 v_1 = \rho_2 A_2 v_2[/tex]
Or, using the ideal gas law
[tex]P_1 A_1 v_1 = P_2 A_2 v_2[/tex]
Now, according to equation A, if [itex]v_2 > v_1[/itex], then [itex]P_1 > P_2[/itex], so Bernoulli's principle holds. However, this is where I'm having a little bit of trouble, in the incompressible case, the decrease in flow area is what caused the increase in velocity (and will always do, according to the continuity equation). In the compressible case (and again, according to the corresponding continuity equation) a decrease in flow area may not always result in an increase in velocity, since we have to take into account the change in density/pressure of the gas. In other words, for this particular system, even if [itex]A_1 > A_2[/itex] will always be true, [itex]v_1[/itex] may be greater or smaller than [itex]v_2[/itex], depending on the nature of the gas and the temperature of the system. Is this right?
Are there any rules of thumb or other relationships between flow area and velocity for compressible flows? I know this might seem like a simple detail but it really caught me off guard.
Also, for the incompressible case, is it okay to use gauge pressures in calculations involving the Bernoulli equation or any other form of the mechanical energy balance?
Thanks in advance for any input!