Mike Dacre
- 19
- 10
I am having some trouble understanding why different sources of bromine radicals supposedly brominate an alkene at different positions. What I mean by this:
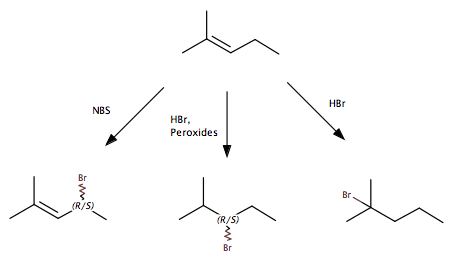 In the first example, the Bromine radical attacks a hydrogen at the allylic position and then a termination reaction results in a Bromine at that position. In the second reaction, the Bromine radical from a split HBr molecule attacks the π bond and adds in an anti-Markovnikov way, because that allows the radical intermediate to be at the more stable tertiary carbon. The final reaction is the non-radical addition of HBr.
In the first example, the Bromine radical attacks a hydrogen at the allylic position and then a termination reaction results in a Bromine at that position. In the second reaction, the Bromine radical from a split HBr molecule attacks the π bond and adds in an anti-Markovnikov way, because that allows the radical intermediate to be at the more stable tertiary carbon. The final reaction is the non-radical addition of HBr.
My question is about the first two reactions here. What favors one over the other. In my mind, both HBr + peroxides and NBS (N-Bromosuccinimide) are a source of Bromine radicals, and the reaction should proceed in much the same way. I know that in the HBr reaction, there are hydrogen radicals floating around also, but I don't think that that is the answer. The mechanisms are different, but I cannot pinpoint what would make one product favored over the others.
I realize that radical reactions are extremely messy, with lots of side products, but the way my textbook (Brown, Foote, Iverson, Anslyn) teaches it it seems like the NBS reaction primarily targets the allylic position and the HBr + peroxides reaction primarily adds to the π bond. Any ideas on what about the mechanism leads to this result would be hugely helpful, I am struggling to find anything useful online (too many competing search results for my search terms).
Thanks!
My question is about the first two reactions here. What favors one over the other. In my mind, both HBr + peroxides and NBS (N-Bromosuccinimide) are a source of Bromine radicals, and the reaction should proceed in much the same way. I know that in the HBr reaction, there are hydrogen radicals floating around also, but I don't think that that is the answer. The mechanisms are different, but I cannot pinpoint what would make one product favored over the others.
I realize that radical reactions are extremely messy, with lots of side products, but the way my textbook (Brown, Foote, Iverson, Anslyn) teaches it it seems like the NBS reaction primarily targets the allylic position and the HBr + peroxides reaction primarily adds to the π bond. Any ideas on what about the mechanism leads to this result would be hugely helpful, I am struggling to find anything useful online (too many competing search results for my search terms).
Thanks!