- #1
Mayhem
- 307
- 196
Out of sheer curiosity, would a polymer of the following structure be theoretically possible?
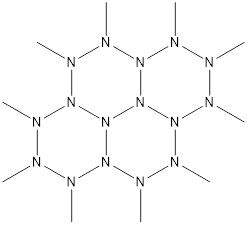
Imagine that the "N-methyl" groups are just the bounds of what is shown, and not actual carbon-containing substituents...
It doesn't seem to break any elementary bonding rules. However, the C-C bond is over twice as strong as the N-N bond, so in practical terms, it would be relatively fragile.
Imagine that the "N-methyl" groups are just the bounds of what is shown, and not actual carbon-containing substituents...
It doesn't seem to break any elementary bonding rules. However, the C-C bond is over twice as strong as the N-N bond, so in practical terms, it would be relatively fragile.
Last edited by a moderator: