bomba923
- 759
- 0
:shy: Just a few simple questions:
1) What medicinal uses does benzhexol have ?
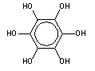
(aka (1,2,3,4,5,6)-hexahydroxybenzene! )
)
2) What is the melting point of benzhexol?
*(see the first GIF image thumbnail for structure)
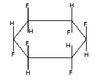
3) What is the melting point of (1,3,5/2,4,6)-hexafluorocyclohexane ?
*(see the third GIF image thumbnail for structure)-
------------------------
4) Generally, how would you synthesize benzhexol, scyllitol, and (1,3,5/2,4,6)-hexafluorocyclohexane? What general mechanisms should I use?
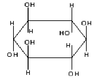
*(see the second GIF image thumbnail for scyllitol structure)
1) What medicinal uses does benzhexol have ?
(aka (1,2,3,4,5,6)-hexahydroxybenzene!
2) What is the melting point of benzhexol?
*(see the first GIF image thumbnail for structure)
3) What is the melting point of (1,3,5/2,4,6)-hexafluorocyclohexane ?
*(see the third GIF image thumbnail for structure)-
------------------------
4) Generally, how would you synthesize benzhexol, scyllitol, and (1,3,5/2,4,6)-hexafluorocyclohexane? What general mechanisms should I use?
*(see the second GIF image thumbnail for scyllitol structure)
Attachments
Last edited: