- #1
flyboy_1234
- 10
- 0
- TL;DR Summary
- Search for method of how to solve for pressure in a column of vapor when the height is large given the state at the top of the column and the height.
Background:
I am seeking the method of solving the following problem. I’m trying to work through a real-world problem and am stumped on P=ρ *g*h when h is large (density changes significantly)(vapor/gas). I tried to isolate the issue from my bigger problem to help find a formulaic way to solve this. I hope I have formed my question correctly and have not contradicted myself in the process. Below I will show why straight P=ρ *g*h does not work and then explain my approach… for your appraisal.
Problem:
Q: What is the pressure at the bottom of a U-Tube?
In the land of Jack and the Bean Stock, we have a U-Tube that is ridged and well-insulated attached to the bean stock with the top at 1,000 m above the ground (bottom at 0 m). The tops of the U-Tube is connected together with a pump moving 1 kg/minute of CO2 vapor. The pressure at the inlet side is 1 bar (100,000 Pa) and the entropy is 2.5 kJ/kg-K. What is the pressure at the bottom? Consider the system as frictionless. The flow is laminar and steady-state.Diagram 1 - Shows State 1, 2, 3
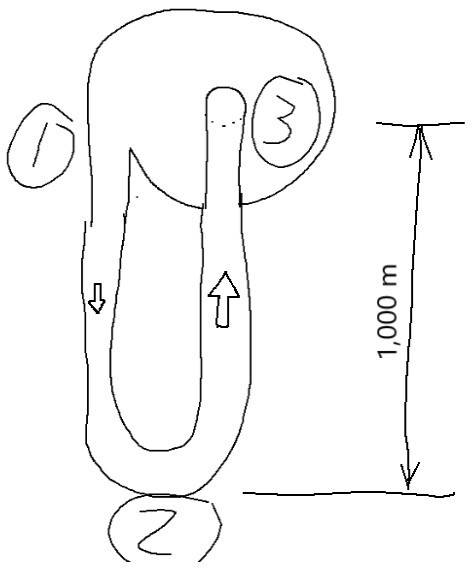
Standard approach (that does not work) of P=ρ*g*h
Substance: CO2
Flow rate: 1 kg/minute
Entropy: 2.5 kJ/kg-K
Height: 1,000 m
P1=1 bar = 1,000 Pa
State 1:
P1 = 1,000 Pa
s1=s2=s3=2.5 kJ/kg-K
From NIST webbook:
ρ1=2.4147 kg/m3
State 2:
Solve for P2
P2=P1 + (ρ1*g*h)
P2=123,664.06 Pa
From NIST webbook:
ρ2=2.8349 kg/m3
State 3:
Note: if we use the same method we should get State 2 = State 3 since we are frictionless and insulated given that adiabatic process is reversible
Solve for P3
P3=P2 - (ρ2*g*h)
P3=95,882.04 Pa <=== Not equal to P1
My approach:
Ideally, I would like to start by using the pressure in the middle then find the pressure at the top and bottom by
P1=Pmiddle - (ρmiddle*g*(1/2)h)
P3=Pmiddle + (ρmiddle*g*(1/2)h)
Since I don't know the middle pressure (or if this is a valid approach) I then have to just go by trial and error until I am close... not exactly an elegant approach.
I am seeking the method of solving the following problem. I’m trying to work through a real-world problem and am stumped on P=ρ *g*h when h is large (density changes significantly)(vapor/gas). I tried to isolate the issue from my bigger problem to help find a formulaic way to solve this. I hope I have formed my question correctly and have not contradicted myself in the process. Below I will show why straight P=ρ *g*h does not work and then explain my approach… for your appraisal.
Problem:
Q: What is the pressure at the bottom of a U-Tube?
In the land of Jack and the Bean Stock, we have a U-Tube that is ridged and well-insulated attached to the bean stock with the top at 1,000 m above the ground (bottom at 0 m). The tops of the U-Tube is connected together with a pump moving 1 kg/minute of CO2 vapor. The pressure at the inlet side is 1 bar (100,000 Pa) and the entropy is 2.5 kJ/kg-K. What is the pressure at the bottom? Consider the system as frictionless. The flow is laminar and steady-state.Diagram 1 - Shows State 1, 2, 3
Standard approach (that does not work) of P=ρ*g*h
Substance: CO2
Flow rate: 1 kg/minute
Entropy: 2.5 kJ/kg-K
Height: 1,000 m
P1=1 bar = 1,000 Pa
State 1:
P1 = 1,000 Pa
s1=s2=s3=2.5 kJ/kg-K
From NIST webbook:
ρ1=2.4147 kg/m3
State 2:
Solve for P2
P2=P1 + (ρ1*g*h)
P2=123,664.06 Pa
From NIST webbook:
ρ2=2.8349 kg/m3
State 3:
Note: if we use the same method we should get State 2 = State 3 since we are frictionless and insulated given that adiabatic process is reversible
Solve for P3
P3=P2 - (ρ2*g*h)
P3=95,882.04 Pa <=== Not equal to P1
My approach:
Ideally, I would like to start by using the pressure in the middle then find the pressure at the top and bottom by
P1=Pmiddle - (ρmiddle*g*(1/2)h)
P3=Pmiddle + (ρmiddle*g*(1/2)h)
Since I don't know the middle pressure (or if this is a valid approach) I then have to just go by trial and error until I am close... not exactly an elegant approach.