- #1
kabtq9s
- 3
- 0
Hello,
This question is based on the passage to the left.
Please I need some one to explain it to me in "baby steps" if possible. I already read another explanation of it .. (posted below the picture) but i still can't understand how the final kinetic energy will be the same as the initial potential energy
especially since the explanation of a smiliar problem on this exams states that "The charged electron particles accelerate in an electric field. The electron starts with a velocity that increases as it approaches the anode through the vacuum. "
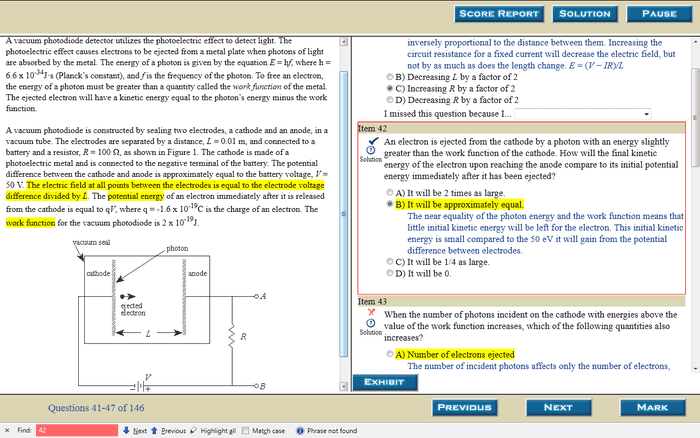
The other explanation: An electron is negatively charged, so it will accelerate from the negatively charged cathode to the positively charged anode. From energy standpoint, this is equivalent to the electron's potential energy being converted to the kinetic energy. If the energy of the photon that is used to eject the electron is only slightly larger than the electron's work function, then the electron has very little kinetic energy. In other words, its initial kinetic energy is approximately 0. When the electron reaches the anode, approximately all of its final kinetic energy is from the initial potential energy. Hence they are equal.
This question is based on the passage to the left.
Please I need some one to explain it to me in "baby steps" if possible. I already read another explanation of it .. (posted below the picture) but i still can't understand how the final kinetic energy will be the same as the initial potential energy
especially since the explanation of a smiliar problem on this exams states that "The charged electron particles accelerate in an electric field. The electron starts with a velocity that increases as it approaches the anode through the vacuum. "
The other explanation: An electron is negatively charged, so it will accelerate from the negatively charged cathode to the positively charged anode. From energy standpoint, this is equivalent to the electron's potential energy being converted to the kinetic energy. If the energy of the photon that is used to eject the electron is only slightly larger than the electron's work function, then the electron has very little kinetic energy. In other words, its initial kinetic energy is approximately 0. When the electron reaches the anode, approximately all of its final kinetic energy is from the initial potential energy. Hence they are equal.