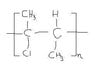
Polymerization of 2-Chloro-2-Butene: Solution
- Thread starter Macroer
- Start date
-
- Tags
- Polymer
Similar threads
Chemistry
Elimination question on alkyl halide
- · Replies 0 ·
- · Replies 2 ·
- · Replies 1 ·
- · Replies 3 ·
- · Replies 2 ·
- · Replies 3 ·
- · Replies 11 ·