- #1
Richie9384
- 4
- 0
I'm having difficulty understanding polymorphs and stacking. I'm doing simulations on 6H-SiC but I think I may have the wrong structure..
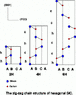
I don't understand the stacking sequence, the picture I attached shows the stacking sequence for 6H-SiC, but I can't make any sense out of it. The arrangement of the A's do not look similar, although B and C seem consistent in terms of the arrangement of atoms.
Basically I've just tried to assemble the unit cell by arranging the atoms in the way shown, the unit cell I used as 12 atoms, I attached a similar picture from the web of the unit cell.
I don't understand the stacking sequence, the picture I attached shows the stacking sequence for 6H-SiC, but I can't make any sense out of it. The arrangement of the A's do not look similar, although B and C seem consistent in terms of the arrangement of atoms.
Basically I've just tried to assemble the unit cell by arranging the atoms in the way shown, the unit cell I used as 12 atoms, I attached a similar picture from the web of the unit cell.