- #1
Richie9384
- 4
- 0
I'm having some difficulty understanding how to assemble a particular polytype of SiC and I think I may have made a mistake...my solid state knowledge is limited.
Basically I start with a hexagonal unit cell for SiC (Moisssanite; four atoms), I attached an image of this unit...I then extended it to build what I think is the unit cell of 6H-SiC (12 atoms)...then I extended the unit cell 5X5X1 (ABC) 6H-SiC so I can run tests on the (0001) and (000-1) surfaces.
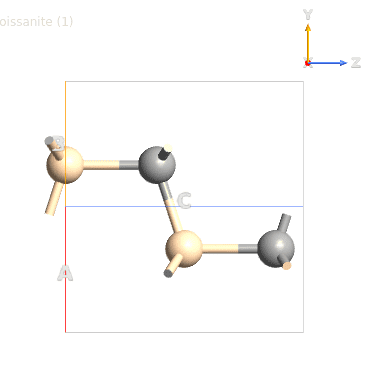
Moissanite Hexagonal SiC
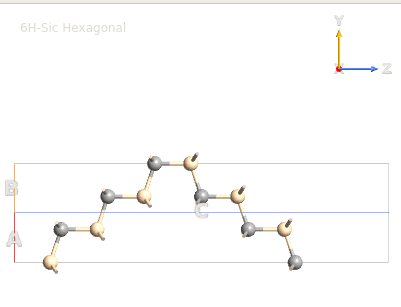
6H-SiC Unit Cell
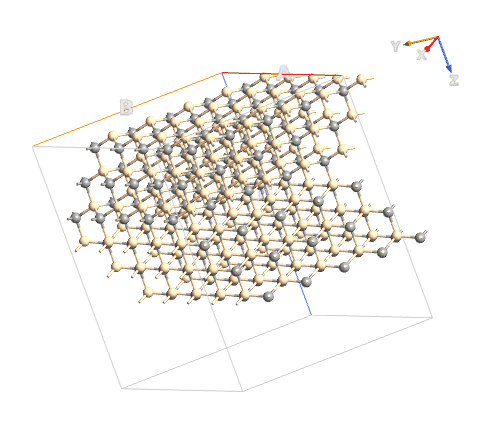
Rotated View of the 5X5X1 Structure
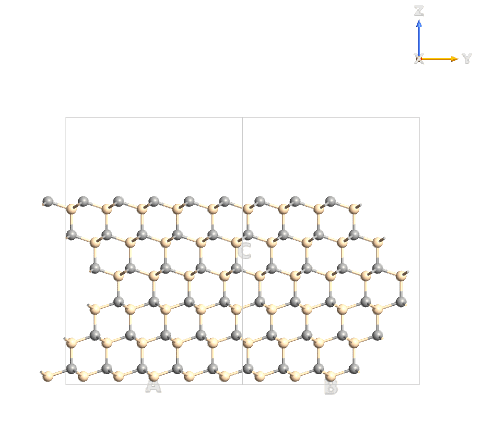
Another view in the yz-plane
Basically I start with a hexagonal unit cell for SiC (Moisssanite; four atoms), I attached an image of this unit...I then extended it to build what I think is the unit cell of 6H-SiC (12 atoms)...then I extended the unit cell 5X5X1 (ABC) 6H-SiC so I can run tests on the (0001) and (000-1) surfaces.
Moissanite Hexagonal SiC
6H-SiC Unit Cell
Rotated View of the 5X5X1 Structure
Another view in the yz-plane