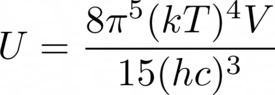- #1
Bookworm092
- 2
- 2
- Homework Statement
- Given the internal energy U of a photon gas, one can take its partial derivative with respect to volume V to get the pressure P (or rather its negative). But if U is calculated by integrating Planck's distribution (see attached), then the result will not be one third of U/V, a result from kinetic theory. Please explain what has gone wrong.
- Relevant Equations
- See attached.
This is from Problem 7.45 of Thermal Physics by Daniel Schroeder.