member 731016
- Homework Statement
- Please see below
- Relevant Equations
- ##\Delta S = \frac{Q}{T}##
For this,
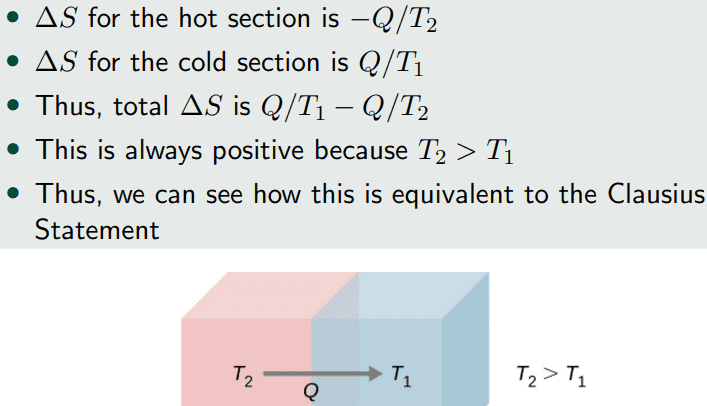
I don't understand how we can apply the change in entropy equation for each solid since the ##\frac{dT}{dt}## for each solid will be non-zero until the solids reach thermal equilibrium. My textbook says that the ##\Delta S## for a system undergoing a reversible process at constant temperature is given by
##\Delta S = \frac{Q}{T}##, however, the temperature of the each solid is not constant while the heat is getting exchanged.
Dose anybody please know what allows them to do that?
Many thanks!
I don't understand how we can apply the change in entropy equation for each solid since the ##\frac{dT}{dt}## for each solid will be non-zero until the solids reach thermal equilibrium. My textbook says that the ##\Delta S## for a system undergoing a reversible process at constant temperature is given by
##\Delta S = \frac{Q}{T}##, however, the temperature of the each solid is not constant while the heat is getting exchanged.
Dose anybody please know what allows them to do that?
Many thanks!