currently
- 21
- 5
Summary:: How can Schrödinger's Equation be written relative to vacuum permittivity
I am wondering why a particular problem uses this equation:
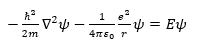
It is stated to be Schrödinger's equation. Where does the potential come in, as well as the e^2/r ?
An explanation would be greatly appreciated. Thanks.
I am wondering why a particular problem uses this equation:
It is stated to be Schrödinger's equation. Where does the potential come in, as well as the e^2/r ?
An explanation would be greatly appreciated. Thanks.