- #1
ChiralSuperfields
- 1,206
- 132
- Homework Statement
- I am confused why the answer to the problem below has more significant figures than it should.
- Relevant Equations
- ##\Delta E_{int} = Q - W##
For this problem,
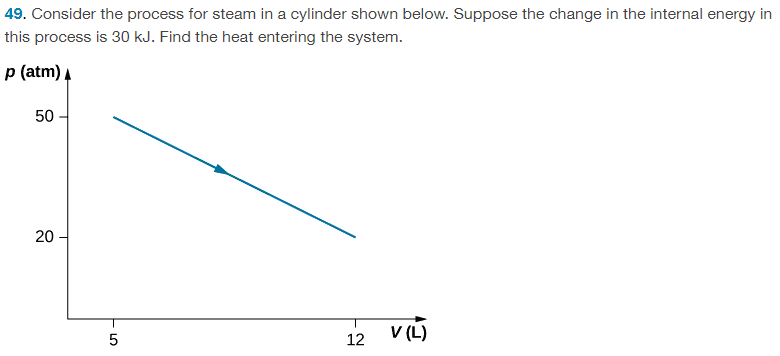
The solution is, ## Q = 54500 J ## , however, since the calculation for heat uses the first law which involves ##5 \times 10^{-3) m^3 ## value for the initial volume, should the finial answer not be to 1 sig fig as well? This would give ## 60000 J ##.
Many thanks!
The solution is, ## Q = 54500 J ## , however, since the calculation for heat uses the first law which involves ##5 \times 10^{-3) m^3 ## value for the initial volume, should the finial answer not be to 1 sig fig as well? This would give ## 60000 J ##.
Many thanks!