- #1
FranzDiCoccio
- 342
- 41
Hi,
I was just wondering about the efficiency of a cycle that is not Carnot cycle.
In that case one should use [itex]\eta = 1-\left|\frac{Q_{\rm out}}{Q_{\rm in}}\right|[/itex], where [itex]Q_{\rm in}[/itex] and [itex]Q_{\rm out}[/itex] are the amounts of heat absorbed and released during the cycle.
For instance, I guess that in an (ideal) Stirling cycle (isochoric - isothermal expansion - isochoric - isothermal compression) [itex]Q_{\rm in}[/itex] involves the isochoric transformation with increasing temperature and the isothermal expansion, while [itex]Q_{\rm out}[/itex] involves the remaining two transformations.
I quickly browsed online and I actually found something like that.
Now the usual diagram for a generic machine (at least in my textbook) seems to suggest that [itex]Q_{\rm in}[/itex] is absorbed from a heat resevoir at some temperature [itex]T_h[/itex] and [itex]Q_{\rm out}[/itex] released into a heat sink at a lower temperature [itex]T_c<T_h[/itex].
See e.g. this one I found on the web, which is similar to the ones in my textbook
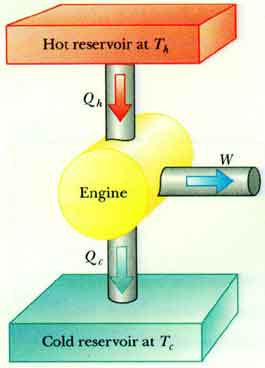 But, in the case of the Stirling cycle, what does it really mean that [itex]Q_{\rm in}=Q_{\rm isothermal}+Q_{\rm isochoric}[/itex] is absorbed "at [itex]T_h[/itex]"? It seems to me that this could apply to the isothermal part only, because the heat intake in the isochoric process does not happen at a fixed temperature...Are all of these diagrams actually referring to a Carnot engine?
But, in the case of the Stirling cycle, what does it really mean that [itex]Q_{\rm in}=Q_{\rm isothermal}+Q_{\rm isochoric}[/itex] is absorbed "at [itex]T_h[/itex]"? It seems to me that this could apply to the isothermal part only, because the heat intake in the isochoric process does not happen at a fixed temperature...Are all of these diagrams actually referring to a Carnot engine?
Or perhaps this has to do with the fact that any cycle can be decomposed into many Carnot cycles, so ultimately the diagrams assume that the heat exchanges happen at fixed temperatures?
Thanks a lot for any insight.
Franz
I was just wondering about the efficiency of a cycle that is not Carnot cycle.
In that case one should use [itex]\eta = 1-\left|\frac{Q_{\rm out}}{Q_{\rm in}}\right|[/itex], where [itex]Q_{\rm in}[/itex] and [itex]Q_{\rm out}[/itex] are the amounts of heat absorbed and released during the cycle.
For instance, I guess that in an (ideal) Stirling cycle (isochoric - isothermal expansion - isochoric - isothermal compression) [itex]Q_{\rm in}[/itex] involves the isochoric transformation with increasing temperature and the isothermal expansion, while [itex]Q_{\rm out}[/itex] involves the remaining two transformations.
I quickly browsed online and I actually found something like that.
Now the usual diagram for a generic machine (at least in my textbook) seems to suggest that [itex]Q_{\rm in}[/itex] is absorbed from a heat resevoir at some temperature [itex]T_h[/itex] and [itex]Q_{\rm out}[/itex] released into a heat sink at a lower temperature [itex]T_c<T_h[/itex].
See e.g. this one I found on the web, which is similar to the ones in my textbook
Or perhaps this has to do with the fact that any cycle can be decomposed into many Carnot cycles, so ultimately the diagrams assume that the heat exchanges happen at fixed temperatures?
Thanks a lot for any insight.
Franz