- #1
Soren4
- 128
- 2
I'm confused on the efficiency of a Thermal engine in the case it is reversible or not reversible, in particular where the ideal gas follows isochoric or isobaric processes.
Infact during isochoric and isobaric processes $$Q_{isochoric}=n c_v \Delta T$$
$$Q_{isobaric}=n c_p \Delta T$$
So the heats depend only on the temperatures and not on the process. So it really seems to me that the reversibility or irreversibility does not change the efficiency at all. Consider the cycle in the diagram, made of 2 isochoric and 2 isobaric processes. The cycle is clockwise.
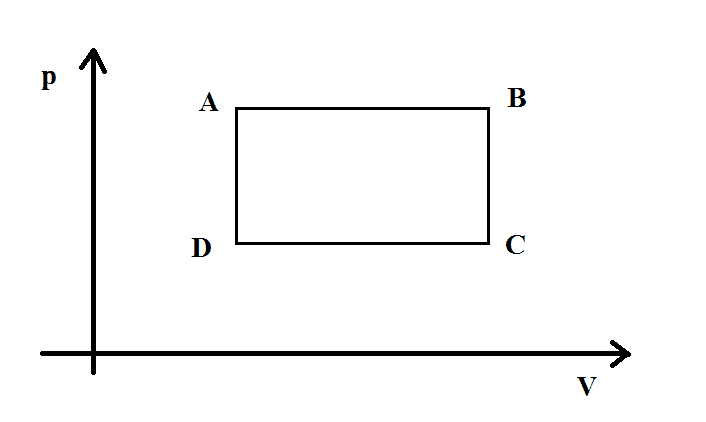
The efficiency is $$\eta=1+\frac{|Q_{C->D}+Q_{B->C}|}{Q_{A->B}+Q_{D->A}}=1+\frac{|c_p(T_{D}-T_{C})+c_v(T_{C}-T_{B})|}{c_p(T_{B}-T_{A})+c_v(T_{A}-T_{D})}\tag{I}$$
Consider the two following cases
On the one hand my answer would be no, as said before, because heats are all functions of temperatures only.
On the other hand this does not makes sense, because Carnot theorem requires that in case 1 (all processes reversible) the efficiency is the same of a Carnot engine working betweeen highest and lowest temperature (in this case ##T_{D}## and ##T_{B}##), that is, in case one, $$\eta=1-\frac{T_D}{T_B}\tag{II}$$
Which is not equal to ##(I)##.
To sum up, it seems to me that, in this case, ##\eta## does not depend on the reversibility of the engine. I don't see this dipendence which of course is there, because of Carnot theorem. So how does ##\eta## depend of the reversibility of processes in this one case?
Thanks a lot in advice
Infact during isochoric and isobaric processes $$Q_{isochoric}=n c_v \Delta T$$
$$Q_{isobaric}=n c_p \Delta T$$
So the heats depend only on the temperatures and not on the process. So it really seems to me that the reversibility or irreversibility does not change the efficiency at all. Consider the cycle in the diagram, made of 2 isochoric and 2 isobaric processes. The cycle is clockwise.
The efficiency is $$\eta=1+\frac{|Q_{C->D}+Q_{B->C}|}{Q_{A->B}+Q_{D->A}}=1+\frac{|c_p(T_{D}-T_{C})+c_v(T_{C}-T_{B})|}{c_p(T_{B}-T_{A})+c_v(T_{A}-T_{D})}\tag{I}$$
Consider the two following cases
- All the processes in the diagram are reversible
- One of more of the processes is not reversible
On the one hand my answer would be no, as said before, because heats are all functions of temperatures only.
On the other hand this does not makes sense, because Carnot theorem requires that in case 1 (all processes reversible) the efficiency is the same of a Carnot engine working betweeen highest and lowest temperature (in this case ##T_{D}## and ##T_{B}##), that is, in case one, $$\eta=1-\frac{T_D}{T_B}\tag{II}$$
Which is not equal to ##(I)##.
To sum up, it seems to me that, in this case, ##\eta## does not depend on the reversibility of the engine. I don't see this dipendence which of course is there, because of Carnot theorem. So how does ##\eta## depend of the reversibility of processes in this one case?
Thanks a lot in advice