Ravey
- 5
- 0
1. What is the thermal efficiency for the heat engine shown in the figure?
What is the heat extracted from the hot reservoir for the heat engine shown in the figure?t
Figure shown here:
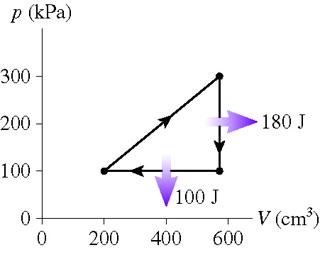
2.Wout = 1/2 B*H Also, Qc=Qh-Wout, efficiency: Wout/Qh
3. Wout = .5 * 200KPA* (400x10^-6m^3). I have no idea how to obtain Qc (cold reservoir) or Qh (hot)
What is the heat extracted from the hot reservoir for the heat engine shown in the figure?t
Figure shown here:
2.Wout = 1/2 B*H Also, Qc=Qh-Wout, efficiency: Wout/Qh
3. Wout = .5 * 200KPA* (400x10^-6m^3). I have no idea how to obtain Qc (cold reservoir) or Qh (hot)