adityax26
- 7
- 0
Member advised to use the formatting template for all homework help requests
Hey all, I'm new to the forums and had a quick question:
(please tell me if this is the wrong topic under which I posted, please move it if needed! @mods)
I am doing a lab report investigating the effect of temperature (˚C) on the dynamic viscosity (Pa s) of glycerin. I am timing the time it takes for a small sphere (marble) to travel from point A to point B with terminal velocity and plugging this into the Stoke's Law Equation solving for viscosity. I am testing: 20 ˚C, 30 ˚C, 40 ˚C, 50 ˚C, 60 ˚C, 70 ˚C, and 80 ˚C. I had a few questions I'd like to ask if you guys!
1) How do I know that the marble when dropped will reach terminal velocity when dropping from a certain point to another? I am writing my controlled variables, and this is one of them. Is there an equation I can use with drag or something to find the time/distance required to reach this value? Essentially I tried the experiment with sunflower oil & water, but those were WAY too less viscous and it dropped within a few milliseconds; hard to measure, not sure if it even dropped at terminal velocity...
2) Is glycerin (or glycerol, same thing) a good liquid to use for such experiment? I am thinking of using either 1L or 2L of glycerin. I can let the marble drop for a maximum of 10-15 cm, and by then it MUST HAVE reached terminal velocity; do you think it will do so? Do you reccomend using 2L glycerin or will 1 L do? Will I also have enough time to measure within the two points of measurements (I will measure the time it takes to travel like 20 cm at terminal velocity)? The diameter of my tubes are like 6-7 cm and my marble diameter is like 1.5 cm (with mass of around 15 g)
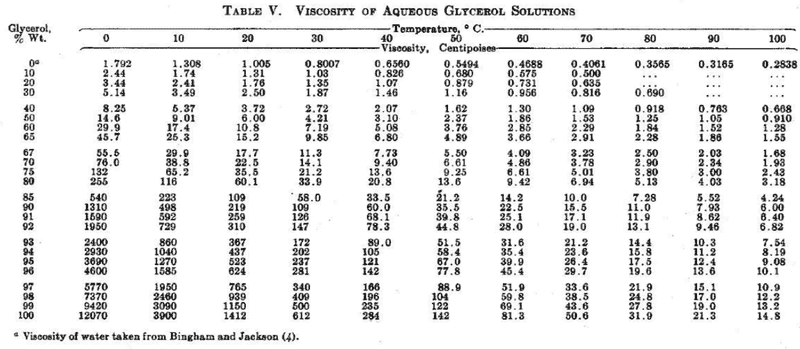
3) When looking at some existing data, I noticed that temp. and viscosity are are inversely proportional. I took some data from the Internet, but how do I linearise this? In theory I thought that graphing a 1/T graph vs. viscosity would achieve this, but I don't really get a linear graph, do I? My two graphs:
https://imgur.com/uPWAhmG
https://imgur.com/eRBAgpE
Glycerin & its viscosity at different temps/concs (I am using 100% vegetable glycerine):
Thanks so much! :D
(please tell me if this is the wrong topic under which I posted, please move it if needed! @mods)
I am doing a lab report investigating the effect of temperature (˚C) on the dynamic viscosity (Pa s) of glycerin. I am timing the time it takes for a small sphere (marble) to travel from point A to point B with terminal velocity and plugging this into the Stoke's Law Equation solving for viscosity. I am testing: 20 ˚C, 30 ˚C, 40 ˚C, 50 ˚C, 60 ˚C, 70 ˚C, and 80 ˚C. I had a few questions I'd like to ask if you guys!
1) How do I know that the marble when dropped will reach terminal velocity when dropping from a certain point to another? I am writing my controlled variables, and this is one of them. Is there an equation I can use with drag or something to find the time/distance required to reach this value? Essentially I tried the experiment with sunflower oil & water, but those were WAY too less viscous and it dropped within a few milliseconds; hard to measure, not sure if it even dropped at terminal velocity...
2) Is glycerin (or glycerol, same thing) a good liquid to use for such experiment? I am thinking of using either 1L or 2L of glycerin. I can let the marble drop for a maximum of 10-15 cm, and by then it MUST HAVE reached terminal velocity; do you think it will do so? Do you reccomend using 2L glycerin or will 1 L do? Will I also have enough time to measure within the two points of measurements (I will measure the time it takes to travel like 20 cm at terminal velocity)? The diameter of my tubes are like 6-7 cm and my marble diameter is like 1.5 cm (with mass of around 15 g)
3) When looking at some existing data, I noticed that temp. and viscosity are are inversely proportional. I took some data from the Internet, but how do I linearise this? In theory I thought that graphing a 1/T graph vs. viscosity would achieve this, but I don't really get a linear graph, do I? My two graphs:
https://imgur.com/uPWAhmG
https://imgur.com/eRBAgpE
Glycerin & its viscosity at different temps/concs (I am using 100% vegetable glycerine):
Thanks so much! :D