- #1
etotheipi
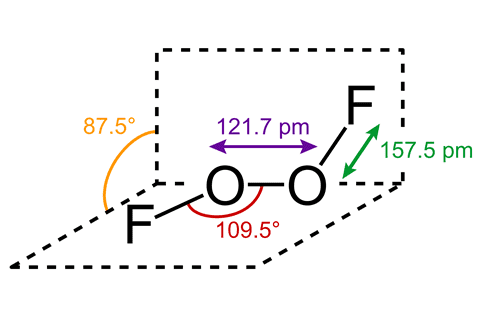
FOOF looks like this:
This paper suggests that FOOOF also exists but does not give a structure, although it does state that it's unlikely it takes the F-O-O-O-F structure. "Ignition: An informal history of liquid rocket propellants" claims that this series of compounds, up to FOOOOOOF, can exist.
How can we go about finding the structure of these more exotic species? Thanks!
This paper suggests that FOOOF also exists but does not give a structure, although it does state that it's unlikely it takes the F-O-O-O-F structure. "Ignition: An informal history of liquid rocket propellants" claims that this series of compounds, up to FOOOOOOF, can exist.
How can we go about finding the structure of these more exotic species? Thanks!