mstud
- 69
- 0
Homework Statement
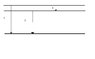
The figure (see attachment) shows part of a energy-state-diagram for an atom. Three energy states are included. At energy transition 1 & 2 the atom emit radiation with the wavelengths of 2.56 \cdot 10^{-8} m and 3.04 \cdot 10^{-8} m, respectively.
Find the wavelength for the radiation which is emitted at transition 3.
Homework Equations
N/A
The Attempt at a Solution
I tried to take wavelength 1 minus wavelength 2, but this gave a completely wrong answer.
I got 4.8 nm. But the answer should be 162 nm.
How shall I then solve it? Does it make any difference to calculate the energy of the two transitions and find the difference between them, or will that give me the same weird answer?
ANY ideas?
Thanks