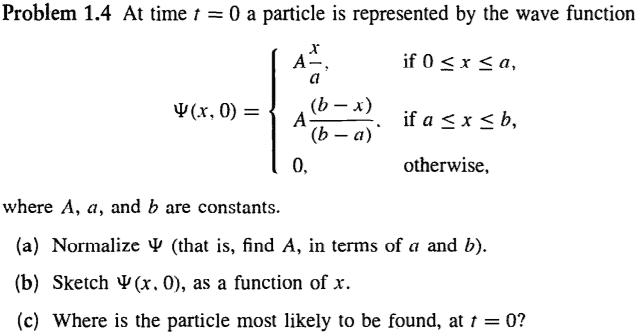blackbeans
- 3
- 0
- Homework Statement
- Hi there, it seems more convenient to post a picture of the problem in question. More specifically, problem 1(c).
- Relevant Equations
- the schrodinger equation.
The wave function described seems impossible. Wave functions have to be differentiable at all points, right? Otherwise they don't represent a physically realizable state. The wave function in the example isn't differentiable at x=A, the maximum point. Also, for problem (c), I know it's visually simple to see the answer, but for a more general case, how would i find the coordinate where the "particle is most likely to be"? Would I take the derivative of |psi|^2 or |psi| to find the absolute maxima? Do they provide the same result? Is there a simpler method?