unscientific
- 1,728
- 13
Both ##^{87}_{37}Rb## and ##^{87}_{38}Sr## are odd-even nuclei, so we can ignore the pairing term ##\delta##. I tried to calculate the most stable Z for a given A by finding ##\frac{\partial B}{\partial Z} = 0##. That gives the most Z-stable value of ##Z_0 = \frac{2\gamma A}{4\gamma + \epsilon A^{\frac{2}{3}}}## which is ##38## for ##A=87##.
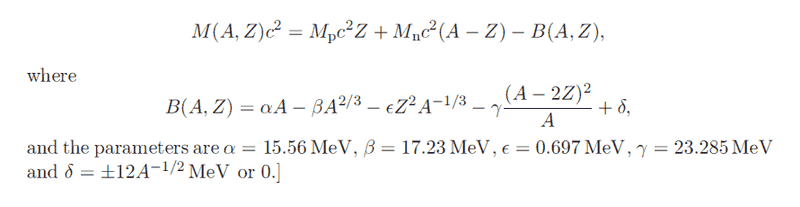
If that's the case, then why wouldn't Rb beta decay to Strontium as these are naturally occurring isobars.
If that's the case, then why wouldn't Rb beta decay to Strontium as these are naturally occurring isobars.